

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50518789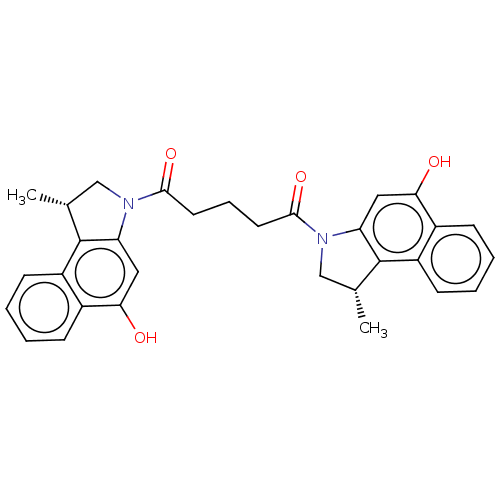 (CHEMBL4473548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged VEGFR2 (D807 to V1356 residues) expressed in sf9 cells using Poly(Glu,Tyr) 4:1 as substrate aft... | J Nat Prod 82: 16-26 (2019) Article DOI: 10.1021/acs.jnatprod.8b00233 BindingDB Entry DOI: 10.7270/Q2FX7DVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alcohol dehydrogenase 1A (Homo sapiens (Human)) | BDBM50518790 (CHEMBL4465918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of ADH1 (unknown origin) preincubated for 2 hrs followed by substrate/NAD+ addition | J Nat Prod 82: 16-26 (2019) Article DOI: 10.1021/acs.jnatprod.8b00233 BindingDB Entry DOI: 10.7270/Q2FX7DVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50518790 (CHEMBL4465918) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged VEGFR2 (D807 to V1356 residues) expressed in sf9 cells using Poly(Glu,Tyr) 4:1 as substrate aft... | J Nat Prod 82: 16-26 (2019) Article DOI: 10.1021/acs.jnatprod.8b00233 BindingDB Entry DOI: 10.7270/Q2FX7DVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50375784 (CHEMBL408621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universit£t Freiburg Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase | Bioorg Med Chem 16: 2385-90 (2008) Article DOI: 10.1016/j.bmc.2007.11.070 BindingDB Entry DOI: 10.7270/Q2CC11K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL2 (Homo sapiens (Human)) | BDBM50518789 (CHEMBL4473548) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST/His6-tagged ABL2 (M1 to P650 residues) expressed in sf9 cells using Poly(Glu,Tyr) 4:1 as substrate aft... | J Nat Prod 82: 16-26 (2019) Article DOI: 10.1021/acs.jnatprod.8b00233 BindingDB Entry DOI: 10.7270/Q2FX7DVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL2 (Homo sapiens (Human)) | BDBM50518790 (CHEMBL4465918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 685 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST/His6-tagged ABL2 (M1 to P650 residues) expressed in sf9 cells using Poly(Glu,Tyr) 4:1 as substrate aft... | J Nat Prod 82: 16-26 (2019) Article DOI: 10.1021/acs.jnatprod.8b00233 BindingDB Entry DOI: 10.7270/Q2FX7DVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50518790 (CHEMBL4465918) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged VEGFR1 (K784 to I1338 residues) expressed in sf9 cells using Poly(Glu,Tyr) 4:1 as substrate aft... | J Nat Prod 82: 16-26 (2019) Article DOI: 10.1021/acs.jnatprod.8b00233 BindingDB Entry DOI: 10.7270/Q2FX7DVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50153015 ((-)-Epicatechin-3-gallate | (-)-epicatechin 3-O-ga...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universita£t Curated by ChEMBL | Assay Description Inhibition of p38alpha after 1 hr by ELISA | J Nat Prod 73: 2035-41 (2010) Article DOI: 10.1021/np100523s BindingDB Entry DOI: 10.7270/Q29P31XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50375785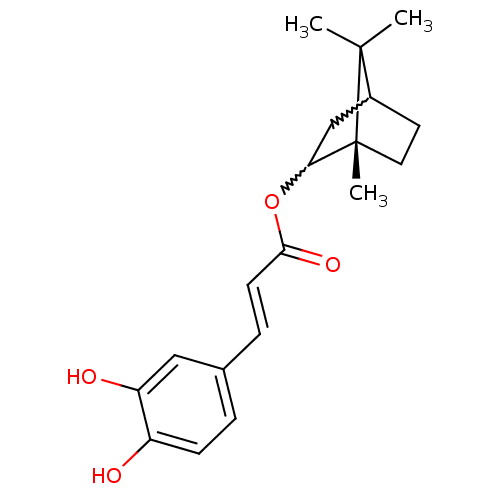 (CHEMBL261166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universit£t Freiburg Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase | Bioorg Med Chem 16: 2385-90 (2008) Article DOI: 10.1016/j.bmc.2007.11.070 BindingDB Entry DOI: 10.7270/Q2CC11K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50518789 (CHEMBL4473548) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged VEGFR1 (K784 to I1338 residues) expressed in sf9 cells using Poly(Glu,Tyr) 4:1 as substrate aft... | J Nat Prod 82: 16-26 (2019) Article DOI: 10.1021/acs.jnatprod.8b00233 BindingDB Entry DOI: 10.7270/Q2FX7DVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM84980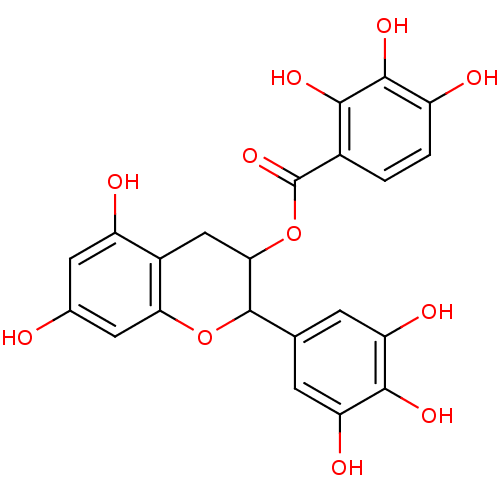 ((-) EPIGALLOCATECHIN GALLATE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50070942 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universita£t Curated by ChEMBL | Assay Description Inhibition of p38alpha after 1 hr by ELISA | J Nat Prod 73: 2035-41 (2010) Article DOI: 10.1021/np100523s BindingDB Entry DOI: 10.7270/Q29P31XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM84980 ((-) EPIGALLOCATECHIN GALLATE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50241242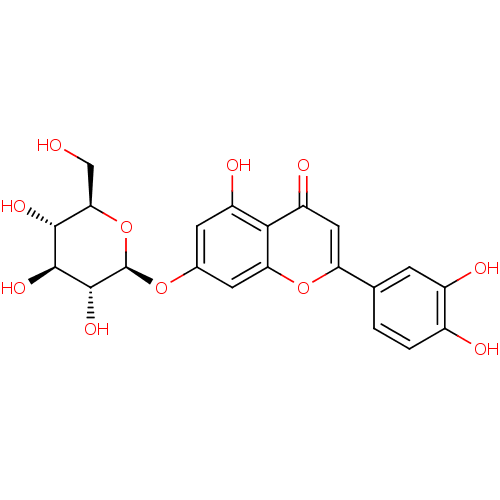 (2-(3,4-dihydroxyphenyl)-5-hydroxy-4-oxo-4H-chromen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM7457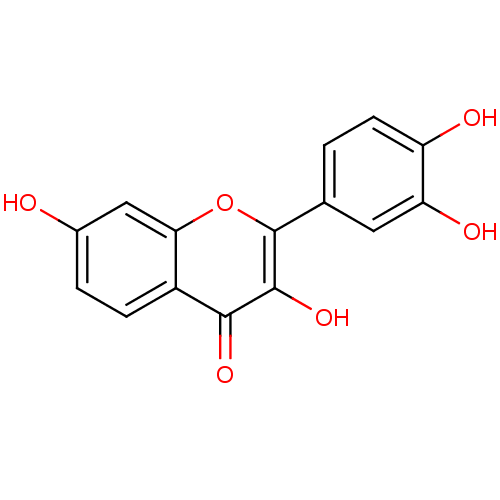 (2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-4H-chromen-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM7457 (2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-4H-chromen-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50518790 (CHEMBL4465918) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST/His6-tagged c-KIT (T544 to V976 residues) expressed in sf9 cells using Poly(Glu,Tyr) 4:1 as substrate ... | J Nat Prod 82: 16-26 (2019) Article DOI: 10.1021/acs.jnatprod.8b00233 BindingDB Entry DOI: 10.7270/Q2FX7DVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50135165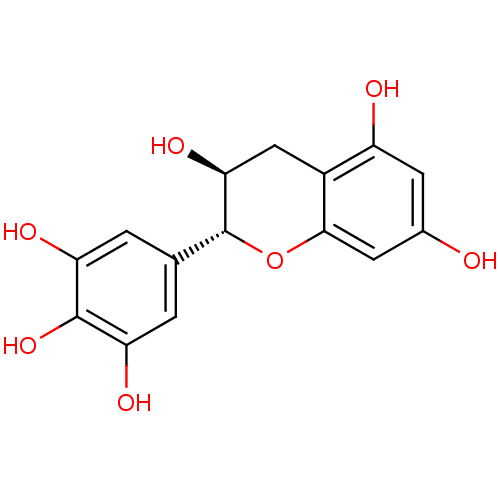 ((+)-gallocatechin | (2R,3S)-2-(3,4,5-Trihydroxy-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50240614 (3,5-Dihydroxy-2-(3-hydroxy-4-methoxy-phenyl)-7-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50518790 (CHEMBL4465918) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST/His6-tagged VEGFR3 (N799 to R1298 residues) expressed in sf9 cells using Poly(Glu,Tyr) 4:1 as substrat... | J Nat Prod 82: 16-26 (2019) Article DOI: 10.1021/acs.jnatprod.8b00233 BindingDB Entry DOI: 10.7270/Q2FX7DVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50135165 ((+)-gallocatechin | (2R,3S)-2-(3,4,5-Trihydroxy-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alcohol dehydrogenase 1A (Homo sapiens (Human)) | BDBM50518789 (CHEMBL4473548) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of ADH1 (unknown origin) preincubated for 2 hrs followed by substrate/NAD+ addition | J Nat Prod 82: 16-26 (2019) Article DOI: 10.1021/acs.jnatprod.8b00233 BindingDB Entry DOI: 10.7270/Q2FX7DVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM23415 (5,7-dihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.78E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM23409 (3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (Rattus norvegicus) | BDBM50409816 (ABAMECTIN) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of Wistar rat heart SERCA2a after 1 hr | J Nat Prod 78: 1262-70 (2015) Article DOI: 10.1021/acs.jnatprod.5b00062 BindingDB Entry DOI: 10.7270/Q2833TR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50240897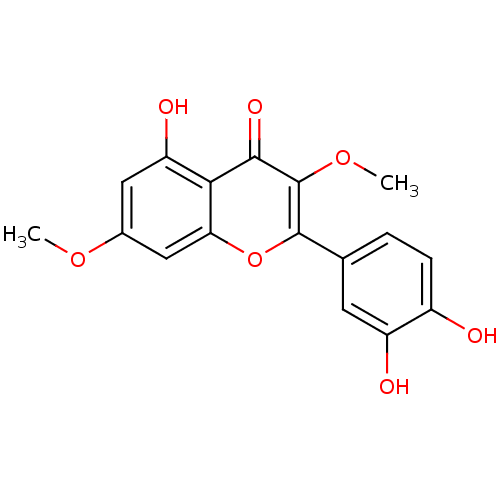 (2-(3,4-dihydroxyphenyl)-5-hydroxy-3,7-dimethoxy-4H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM84983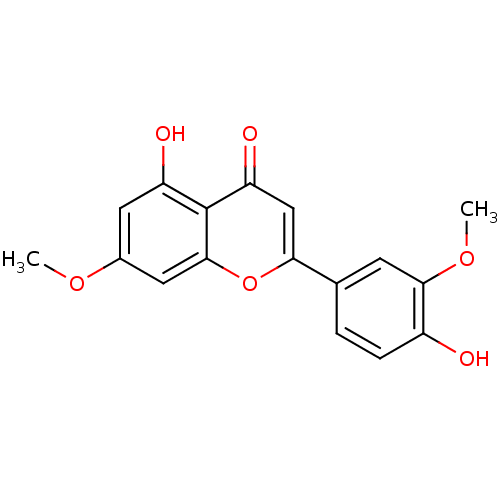 (Velutin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/potassium-transporting ATPase subunit alpha-1 (RAT) | BDBM50409816 (ABAMECTIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of Wistar rat kidney Na+,K+-ATPase alpha1 after 2 hrs | J Nat Prod 78: 1262-70 (2015) Article DOI: 10.1021/acs.jnatprod.5b00062 BindingDB Entry DOI: 10.7270/Q2833TR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50518789 (CHEMBL4473548) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST/His6-tagged c-KIT (T544 to V976 residues) expressed in sf9 cells using Poly(Glu,Tyr) 4:1 as substrate ... | J Nat Prod 82: 16-26 (2019) Article DOI: 10.1021/acs.jnatprod.8b00233 BindingDB Entry DOI: 10.7270/Q2FX7DVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50518789 (CHEMBL4473548) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST/His6-tagged VEGFR3 (N799 to R1298 residues) expressed in sf9 cells using Poly(Glu,Tyr) 4:1 as substrat... | J Nat Prod 82: 16-26 (2019) Article DOI: 10.1021/acs.jnatprod.8b00233 BindingDB Entry DOI: 10.7270/Q2FX7DVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM26658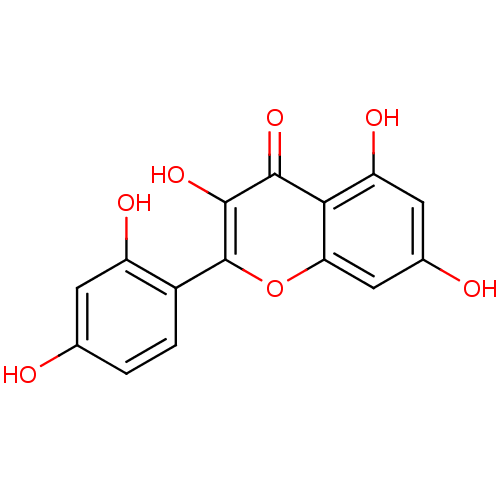 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM7461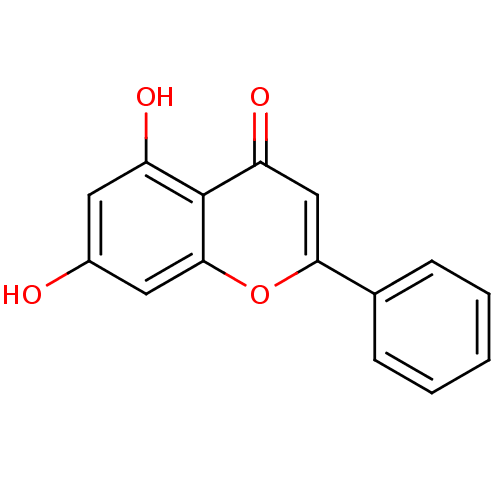 (5,7-dihydroxy-2-phenyl-4H-chromen-4-one | 5,7-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50240897 (2-(3,4-dihydroxyphenyl)-5-hydroxy-3,7-dimethoxy-4H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM23412 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-6-methoxy-4H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM84985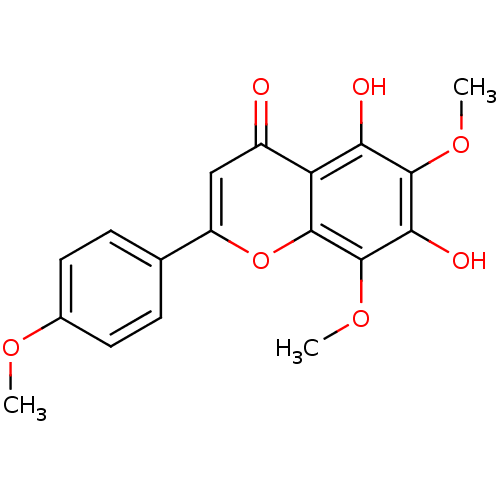 (Nevadensin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM84976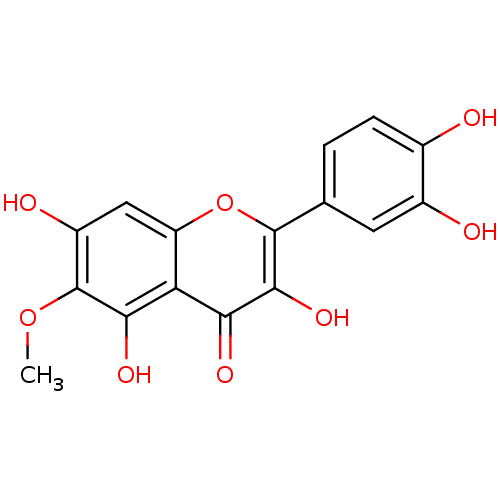 (Patuletin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sarcoplasmic/endoplasmic reticulum calcium ATPase 1 (Rattus norvegicus) | BDBM50409816 (ABAMECTIN) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Ludwigs University Freiburg Curated by ChEMBL | Assay Description Inhibition of Wistar rat EDL muscle SERCA1a after 1 hr | J Nat Prod 78: 1262-70 (2015) Article DOI: 10.1021/acs.jnatprod.5b00062 BindingDB Entry DOI: 10.7270/Q2833TR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM84977 (6-methoxy kaempferol) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM84984 (Jaceosidin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM84984 (Jaceosidin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102 total ) | Next | Last >> |