 Found 142 hits with Last Name = 'miura' and Initial = 'm'
Found 142 hits with Last Name = 'miura' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM31459

(CHEMBL224485 | substituted biphenyl derivative, 21)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccccc1C(=O)Nc1ccc(cc1)C(N)=N Show InChI InChI=1S/C26H26N4O4/c1-15(2)14-29-24(31)17-9-12-20(22(13-17)26(33)34)19-5-3-4-6-21(19)25(32)30-18-10-7-16(8-11-18)23(27)28/h3-13,15H,14H2,1-2H3,(H3,27,28)(H,29,31)(H,30,32)(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197327
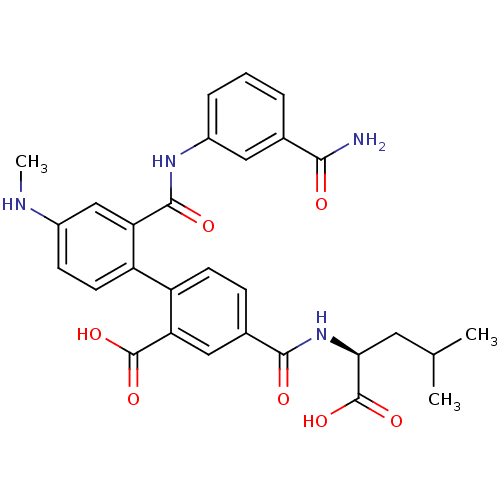
(2'-({[3-(aminocarbonyl)phenyl]amino}carbonyl)-4-{[...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1cccc(c1)C(N)=O)-c1ccc(cc1C(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C29H30N4O7/c1-15(2)11-24(29(39)40)33-26(35)17-7-9-21(23(13-17)28(37)38)20-10-8-18(31-3)14-22(20)27(36)32-19-6-4-5-16(12-19)25(30)34/h4-10,12-15,24,31H,11H2,1-3H3,(H2,30,34)(H,32,36)(H,33,35)(H,37,38)(H,39,40)/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197325
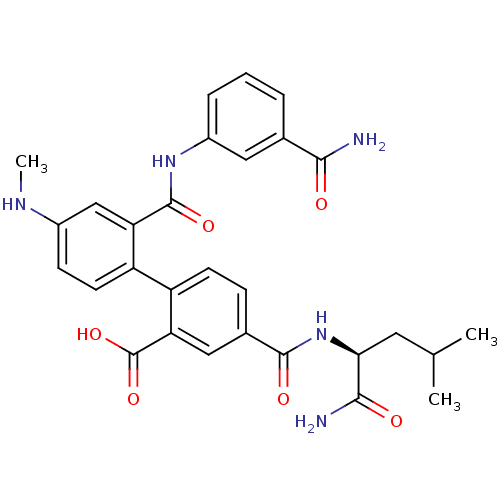
(4-({[(1S)-(aminocarbonyl)-3-methylbutyl]amino}-car...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1cccc(c1)C(N)=O)-c1ccc(cc1C(O)=O)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C29H31N5O6/c1-15(2)11-24(26(31)36)34-27(37)17-7-9-21(23(13-17)29(39)40)20-10-8-18(32-3)14-22(20)28(38)33-19-6-4-5-16(12-19)25(30)35/h4-10,12-15,24,32H,11H2,1-3H3,(H2,30,35)(H2,31,36)(H,33,38)(H,34,37)(H,39,40)/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197331
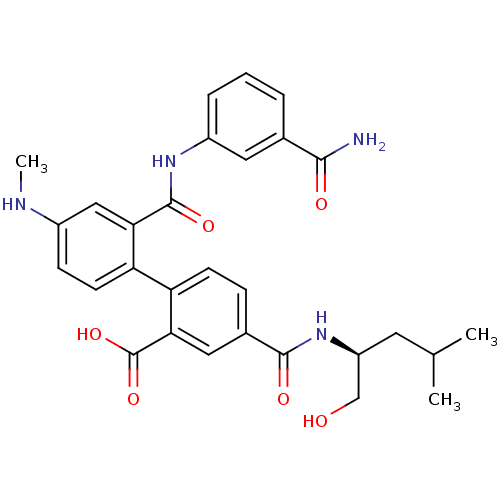
(2'-({[3-(aminocarbonyl)phenyl]amino}carbonyl)-4-({...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1cccc(c1)C(N)=O)-c1ccc(cc1C(O)=O)C(=O)N[C@H](CO)CC(C)C Show InChI InChI=1S/C29H32N4O6/c1-16(2)11-21(15-34)33-27(36)18-7-9-23(25(13-18)29(38)39)22-10-8-19(31-3)14-24(22)28(37)32-20-6-4-5-17(12-20)26(30)35/h4-10,12-14,16,21,31,34H,11,15H2,1-3H3,(H2,30,35)(H,32,37)(H,33,36)(H,38,39)/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197330

(4-({[(1S)-(aminocarbonyl)-3-methylbutyl]amino}-car...)Show SMILES CCNc1ccc(c(c1)C(=O)Nc1cccc(c1)C(N)=O)-c1ccc(cc1C(O)=O)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C30H33N5O6/c1-4-33-19-9-11-21(23(15-19)29(39)34-20-7-5-6-17(13-20)26(31)36)22-10-8-18(14-24(22)30(40)41)28(38)35-25(27(32)37)12-16(2)3/h5-11,13-16,25,33H,4,12H2,1-3H3,(H2,31,36)(H2,32,37)(H,34,39)(H,35,38)(H,40,41)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197324

(4-({[(1S)-(aminocarbonyl)-3-methylbutyl]amino}-car...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1ccc(CN)cc1)-c1ccc(cc1C(O)=O)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C29H33N5O5/c1-16(2)12-25(26(31)35)34-27(36)18-6-10-22(24(13-18)29(38)39)21-11-9-20(32-3)14-23(21)28(37)33-19-7-4-17(15-30)5-8-19/h4-11,13-14,16,25,32H,12,15,30H2,1-3H3,(H2,31,35)(H,33,37)(H,34,36)(H,38,39)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197326

(2'-(3-carbamoyl-phenylcarbamoyl)-4-isobutylcarbamo...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1cccc(c1)C(N)=O)-c1ccc(cc1C(O)=O)C(=O)NCC(C)C Show InChI InChI=1S/C27H28N4O5/c1-15(2)14-30-25(33)17-7-9-21(23(12-17)27(35)36)20-10-8-18(29-3)13-22(20)26(34)31-19-6-4-5-16(11-19)24(28)32/h4-13,15,29H,14H2,1-3H3,(H2,28,32)(H,30,33)(H,31,34)(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197329
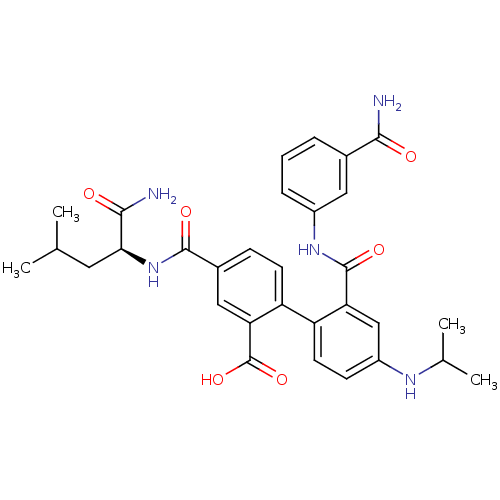
(4-({[(1S)-(aminocarbonyl)-3-methylbutyl]amino}-car...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(NC(C)C)cc1C(=O)Nc1cccc(c1)C(N)=O)C(N)=O Show InChI InChI=1S/C31H35N5O6/c1-16(2)12-26(28(33)38)36-29(39)19-8-10-23(25(14-19)31(41)42)22-11-9-21(34-17(3)4)15-24(22)30(40)35-20-7-5-6-18(13-20)27(32)37/h5-11,13-17,26,34H,12H2,1-4H3,(H2,32,37)(H2,33,38)(H,35,40)(H,36,39)(H,41,42)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM31459

(CHEMBL224485 | substituted biphenyl derivative, 21)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccccc1C(=O)Nc1ccc(cc1)C(N)=N Show InChI InChI=1S/C26H26N4O4/c1-15(2)14-29-24(31)17-9-12-20(22(13-17)26(33)34)19-5-3-4-6-21(19)25(32)30-18-10-7-16(8-11-18)23(27)28/h3-13,15H,14H2,1-2H3,(H3,27,28)(H,29,31)(H,30,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Fxa by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50030766
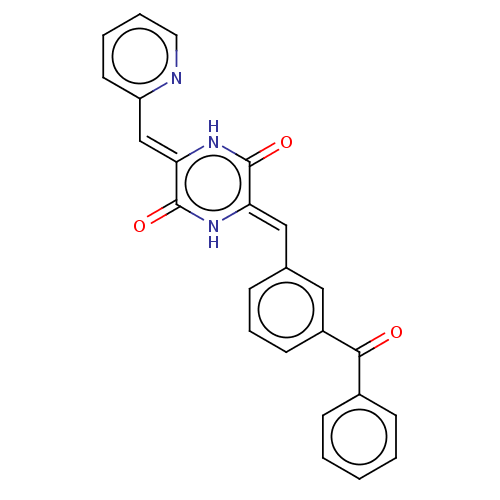
(CHEMBL3342339)Show SMILES O=C(c1ccccc1)c1cccc(\C=c2/[nH]c(=O)\c(=C\c3ccccn3)[nH]c2=O)c1 Show InChI InChI=1S/C24H17N3O3/c28-22(17-8-2-1-3-9-17)18-10-6-7-16(13-18)14-20-23(29)27-21(24(30)26-20)15-19-11-4-5-12-25-19/h1-15H,(H,26,30)(H,27,29)/b20-14-,21-15- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of porcine tubulin polymerization by spectrophotometry |
ACS Med Chem Lett 5: 1094-8 (2014)
Article DOI: 10.1021/ml5001883
BindingDB Entry DOI: 10.7270/Q2R49SCP |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197340

(2'-({[4-(aminomethyl)phenyl]amino}carbonyl)-4-({[(...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1ccc(CN)cc1)-c1ccc(cc1C(O)=O)C(=O)N[C@H](CO)CC(C)C Show InChI InChI=1S/C29H34N4O5/c1-17(2)12-22(16-34)33-27(35)19-6-10-24(26(13-19)29(37)38)23-11-9-21(31-3)14-25(23)28(36)32-20-7-4-18(15-30)5-8-20/h4-11,13-14,17,22,31,34H,12,15-16,30H2,1-3H3,(H,32,36)(H,33,35)(H,37,38)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50030765
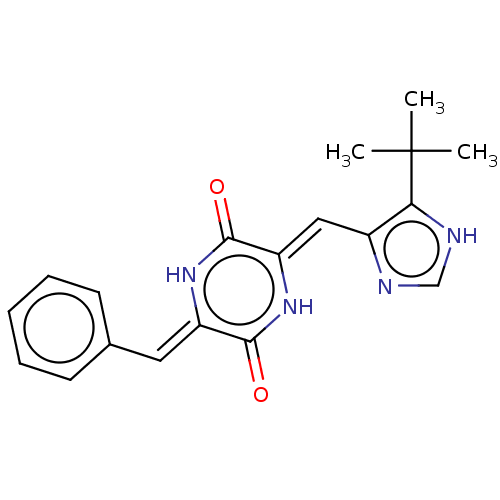
(NPI-2358 | Plinabulin)Show SMILES CC(C)(C)c1[nH]cnc1\C=c1/[nH]c(=O)\c(=C\c2ccccc2)[nH]c1=O Show InChI InChI=1S/C19H20N4O2/c1-19(2,3)16-13(20-11-21-16)10-15-18(25)22-14(17(24)23-15)9-12-7-5-4-6-8-12/h4-11H,1-3H3,(H,20,21)(H,22,25)(H,23,24)/b14-9-,15-10- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of porcine tubulin polymerization by spectrophotometry |
ACS Med Chem Lett 5: 1094-8 (2014)
Article DOI: 10.1021/ml5001883
BindingDB Entry DOI: 10.7270/Q2R49SCP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197336

(4-({[(1S)-(aminocarbonyl)-3-methylbutyl]amino}-car...)Show SMILES CCCCNc1ccc(c(c1)C(=O)Nc1cccc(c1)C(N)=O)-c1ccc(cc1C(O)=O)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C32H37N5O6/c1-4-5-13-35-21-10-12-23(25(17-21)31(41)36-22-8-6-7-19(15-22)28(33)38)24-11-9-20(16-26(24)32(42)43)30(40)37-27(29(34)39)14-18(2)3/h6-12,15-18,27,35H,4-5,13-14H2,1-3H3,(H2,33,38)(H2,34,39)(H,36,41)(H,37,40)(H,42,43)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197341

(2'-({[3-(aminocarbonyl)phenyl]amino}carbonyl)-4-{[...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(c(c1)C(O)=O)-c1ccccc1C(=O)Nc1cccc(c1)C(N)=O)C(O)=O Show InChI InChI=1S/C28H27N3O7/c1-15(2)12-23(28(37)38)31-25(33)17-10-11-20(22(14-17)27(35)36)19-8-3-4-9-21(19)26(34)30-18-7-5-6-16(13-18)24(29)32/h3-11,13-15,23H,12H2,1-2H3,(H2,29,32)(H,30,34)(H,31,33)(H,35,36)(H,37,38)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197333

(2'-({[3-(aminocarbonyl)phenyl]amino}carbonyl)-4-[(...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(c(c1)C(O)=O)-c1ccccc1C(=O)Nc1cccc(c1)C(N)=O)C(=O)N(C)C Show InChI InChI=1S/C30H32N4O6/c1-17(2)14-25(29(38)34(3)4)33-27(36)19-12-13-22(24(16-19)30(39)40)21-10-5-6-11-23(21)28(37)32-20-9-7-8-18(15-20)26(31)35/h5-13,15-17,25H,14H2,1-4H3,(H2,31,35)(H,32,37)(H,33,36)(H,39,40)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197332
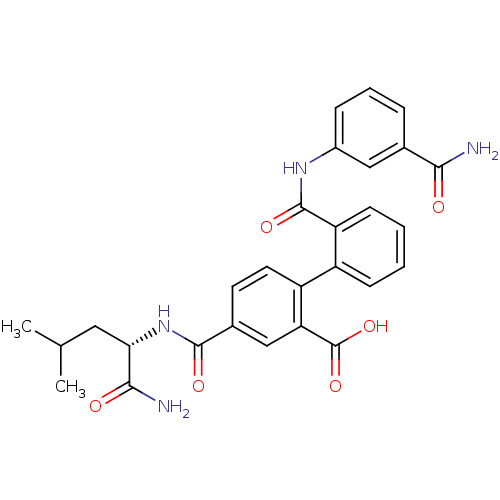
(4-({[(1S)-(aminocarbonyl)-3-methylbutyl]amino}carb...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(c(c1)C(O)=O)-c1ccccc1C(=O)Nc1cccc(c1)C(N)=O)C(N)=O Show InChI InChI=1S/C28H28N4O6/c1-15(2)12-23(25(30)34)32-26(35)17-10-11-20(22(14-17)28(37)38)19-8-3-4-9-21(19)27(36)31-18-7-5-6-16(13-18)24(29)33/h3-11,13-15,23H,12H2,1-2H3,(H2,29,33)(H2,30,34)(H,31,36)(H,32,35)(H,37,38)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197334

(2'-({[3-(aminocarbonyl)phenyl]amino}carbonyl)-4-[(...)Show SMILES CNC(=O)[C@H](CC(C)C)NC(=O)c1ccc(c(c1)C(O)=O)-c1ccccc1C(=O)Nc1cccc(c1)C(N)=O Show InChI InChI=1S/C29H30N4O6/c1-16(2)13-24(28(37)31-3)33-26(35)18-11-12-21(23(15-18)29(38)39)20-9-4-5-10-22(20)27(36)32-19-8-6-7-17(14-19)25(30)34/h4-12,14-16,24H,13H2,1-3H3,(H2,30,34)(H,31,37)(H,32,36)(H,33,35)(H,38,39)/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197338

(2'-({[3-(aminocarbonyl)phenyl]amino}carbonyl)-4-{[...)Show SMILES CC(C)C[C@@H](CO)NC(=O)c1ccc(c(c1)C(O)=O)-c1ccccc1C(=O)Nc1cccc(c1)C(N)=O Show InChI InChI=1S/C28H29N3O6/c1-16(2)12-20(15-32)31-26(34)18-10-11-22(24(14-18)28(36)37)21-8-3-4-9-23(21)27(35)30-19-7-5-6-17(13-19)25(29)33/h3-11,13-14,16,20,32H,12,15H2,1-2H3,(H2,29,33)(H,30,35)(H,31,34)(H,36,37)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197339

(2'-({[3-(aminocarbonyl)phenyl]amino}carbonyl)-4-{[...)Show SMILES CC(C)CCNC(=O)c1ccc(c(c1)C(O)=O)-c1ccccc1C(=O)Nc1cccc(c1)C(N)=O Show InChI InChI=1S/C27H27N3O5/c1-16(2)12-13-29-25(32)18-10-11-21(23(15-18)27(34)35)20-8-3-4-9-22(20)26(33)30-19-7-5-6-17(14-19)24(28)31/h3-11,14-16H,12-13H2,1-2H3,(H2,28,31)(H,29,32)(H,30,33)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197335

(2'-(3-Carbamoyl-phenylcarbamoyl)-4-isobutylcarbamo...)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccccc1C(=O)Nc1cccc(c1)C(N)=O Show InChI InChI=1S/C26H25N3O5/c1-15(2)14-28-24(31)17-10-11-20(22(13-17)26(33)34)19-8-3-4-9-21(19)25(32)29-18-7-5-6-16(12-18)23(27)30/h3-13,15H,14H2,1-2H3,(H2,27,30)(H,28,31)(H,29,32)(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197328
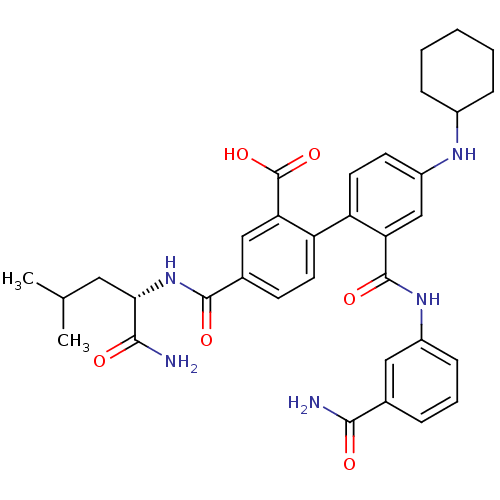
(4-({[(1S)-(aminocarbonyl)-3-methylbutyl]amino}-car...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(NC2CCCCC2)cc1C(=O)Nc1cccc(c1)C(N)=O)C(N)=O Show InChI InChI=1S/C34H39N5O6/c1-19(2)15-29(31(36)41)39-32(42)21-11-13-26(28(17-21)34(44)45)25-14-12-24(37-22-8-4-3-5-9-22)18-27(25)33(43)38-23-10-6-7-20(16-23)30(35)40/h6-7,10-14,16-19,22,29,37H,3-5,8-9,15H2,1-2H3,(H2,35,40)(H2,36,41)(H,38,43)(H,39,42)(H,44,45)/t29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025146
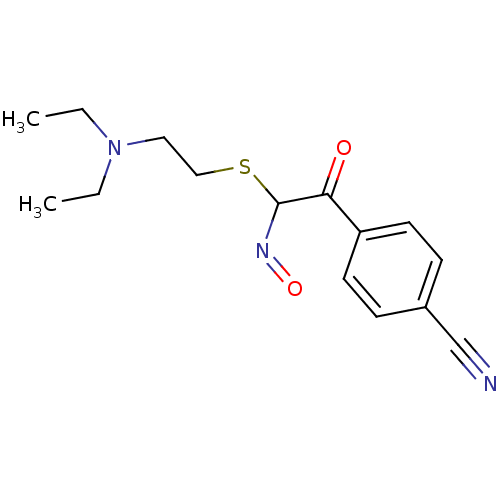
(2-(4-Cyano-phenyl)-N-hydroxy-2-oxo-thioacetimidic ...)Show InChI InChI=1S/C15H19N3O2S/c1-3-18(4-2)9-10-21-15(17-20)14(19)13-7-5-12(11-16)6-8-13/h5-8,15H,3-4,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50197337
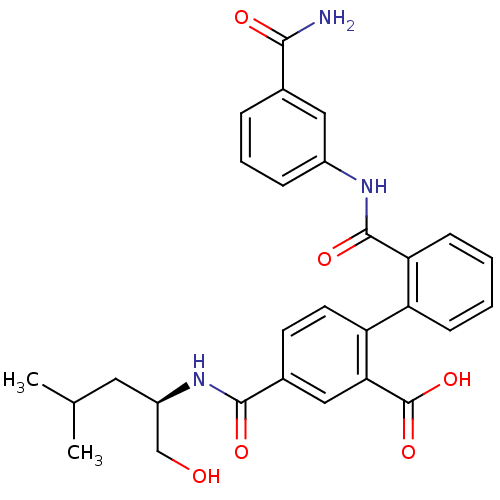
(2'-({[3-(aminocarbonyl)phenyl]amino}carbonyl)-4-({...)Show SMILES CC(C)C[C@H](CO)NC(=O)c1ccc(c(c1)C(O)=O)-c1ccccc1C(=O)Nc1cccc(c1)C(N)=O Show InChI InChI=1S/C28H29N3O6/c1-16(2)12-20(15-32)31-26(34)18-10-11-22(24(14-18)28(36)37)21-8-3-4-9-23(21)27(35)30-19-7-5-6-17(13-19)25(29)33/h3-11,13-14,16,20,32H,12,15H2,1-2H3,(H2,29,33)(H,30,35)(H,31,34)(H,36,37)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.91E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TF/FVIIa complex by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50197326

(2'-(3-carbamoyl-phenylcarbamoyl)-4-isobutylcarbamo...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1cccc(c1)C(N)=O)-c1ccc(cc1C(O)=O)C(=O)NCC(C)C Show InChI InChI=1S/C27H28N4O5/c1-15(2)14-30-25(33)17-7-9-21(23(12-17)27(35)36)20-10-8-18(29-3)13-22(20)26(34)31-19-6-4-5-16(11-19)24(28)32/h4-13,15,29H,14H2,1-3H3,(H2,28,32)(H,30,33)(H,31,34)(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM31459

(CHEMBL224485 | substituted biphenyl derivative, 21)Show SMILES CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccccc1C(=O)Nc1ccc(cc1)C(N)=N Show InChI InChI=1S/C26H26N4O4/c1-15(2)14-29-24(31)17-9-12-20(22(13-17)26(33)34)19-5-3-4-6-21(19)25(32)30-18-10-7-16(8-11-18)23(27)28/h3-13,15H,14H2,1-2H3,(H3,27,28)(H,29,31)(H,30,32)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50197327

(2'-({[3-(aminocarbonyl)phenyl]amino}carbonyl)-4-{[...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1cccc(c1)C(N)=O)-c1ccc(cc1C(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C29H30N4O7/c1-15(2)11-24(29(39)40)33-26(35)17-7-9-21(23(13-17)28(37)38)20-10-8-18(31-3)14-22(20)27(36)32-19-6-4-5-16(12-19)25(30)34/h4-10,12-15,24,31H,11H2,1-3H3,(H2,30,34)(H,32,36)(H,33,35)(H,37,38)(H,39,40)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50197324

(4-({[(1S)-(aminocarbonyl)-3-methylbutyl]amino}-car...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1ccc(CN)cc1)-c1ccc(cc1C(O)=O)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C29H33N5O5/c1-16(2)12-25(26(31)35)34-27(36)18-6-10-22(24(13-18)29(38)39)21-11-9-20(32-3)14-23(21)28(37)33-19-7-4-17(15-30)5-8-19/h4-11,13-14,16,25,32H,12,15,30H2,1-3H3,(H2,31,35)(H,33,37)(H,34,36)(H,38,39)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Fxa by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50197325

(4-({[(1S)-(aminocarbonyl)-3-methylbutyl]amino}-car...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1cccc(c1)C(N)=O)-c1ccc(cc1C(O)=O)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C29H31N5O6/c1-15(2)11-24(26(31)36)34-27(37)17-7-9-21(23(13-17)29(39)40)20-10-8-18(32-3)14-22(20)28(38)33-19-6-4-5-16(12-19)25(30)35/h4-10,12-15,24,32H,11H2,1-3H3,(H2,30,35)(H2,31,36)(H,33,38)(H,34,37)(H,39,40)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Fxa by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50197325

(4-({[(1S)-(aminocarbonyl)-3-methylbutyl]amino}-car...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1cccc(c1)C(N)=O)-c1ccc(cc1C(O)=O)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C29H31N5O6/c1-15(2)11-24(26(31)36)34-27(37)17-7-9-21(23(13-17)29(39)40)20-10-8-18(32-3)14-22(20)28(38)33-19-6-4-5-16(12-19)25(30)35/h4-10,12-15,24,32H,11H2,1-3H3,(H2,30,35)(H2,31,36)(H,33,38)(H,34,37)(H,39,40)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50197324

(4-({[(1S)-(aminocarbonyl)-3-methylbutyl]amino}-car...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1ccc(CN)cc1)-c1ccc(cc1C(O)=O)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C29H33N5O5/c1-16(2)12-25(26(31)35)34-27(36)18-6-10-22(24(13-18)29(38)39)21-11-9-20(32-3)14-23(21)28(37)33-19-7-4-17(15-30)5-8-19/h4-11,13-14,16,25,32H,12,15,30H2,1-3H3,(H2,31,35)(H,33,37)(H,34,36)(H,38,39)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50197326

(2'-(3-carbamoyl-phenylcarbamoyl)-4-isobutylcarbamo...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1cccc(c1)C(N)=O)-c1ccc(cc1C(O)=O)C(=O)NCC(C)C Show InChI InChI=1S/C27H28N4O5/c1-15(2)14-30-25(33)17-7-9-21(23(12-17)27(35)36)20-10-8-18(29-3)13-22(20)26(34)31-19-6-4-5-16(11-19)24(28)32/h4-13,15,29H,14H2,1-3H3,(H2,28,32)(H,30,33)(H,31,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Fxa by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50197327

(2'-({[3-(aminocarbonyl)phenyl]amino}carbonyl)-4-{[...)Show SMILES CNc1ccc(c(c1)C(=O)Nc1cccc(c1)C(N)=O)-c1ccc(cc1C(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C29H30N4O7/c1-15(2)11-24(29(39)40)33-26(35)17-7-9-21(23(13-17)28(37)38)20-10-8-18(31-3)14-22(20)27(36)32-19-6-4-5-16(12-19)25(30)34/h4-10,12-15,24,31H,11H2,1-3H3,(H2,30,34)(H,32,36)(H,33,35)(H,37,38)(H,39,40)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Fxa by chromogenic assay |
Bioorg Med Chem 15: 160-73 (2006)
Article DOI: 10.1016/j.bmc.2006.09.071
BindingDB Entry DOI: 10.7270/Q2MG7P56 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025141

(2-(4-Bromo-phenyl)-N-hydroxy-2-oxo-thioacetimidic ...)Show InChI InChI=1S/C14H19BrN2O2S/c1-3-17(4-2)9-10-20-14(16-19)13(18)11-5-7-12(15)8-6-11/h5-8,14H,3-4,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025142

(CHEMBL46151 | N-Hydroxy-2-(4-methoxy-phenyl)-2-oxo...)Show InChI InChI=1S/C15H22N2O3S/c1-4-17(5-2)10-11-21-15(16-19)14(18)12-6-8-13(20-3)9-7-12/h6-9,15H,4-5,10-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025131

(2-(4-Chloro-phenyl)-N-hydroxy-2-oxo-thioacetimidic...)Show InChI InChI=1S/C16H23ClN2O2S/c1-11(2)19(12(3)4)9-10-22-16(18-21)15(20)13-5-7-14(17)8-6-13/h5-8,11-12,16H,9-10H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50011780
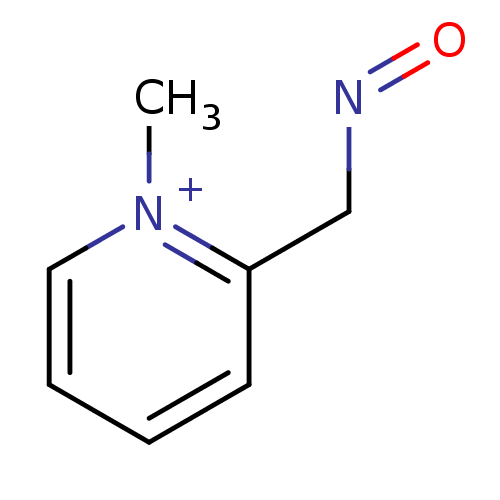
(2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...)Show InChI InChI=1S/C7H9N2O/c1-9-5-3-2-4-7(9)6-8-10/h2-5H,6H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50020522

(1-(((4-carbamoylpyridinium-1-yl)methoxy)methyl)-2-...)Show InChI InChI=1S/C14H15N4O3/c15-14(19)12-4-7-17(8-5-12)10-21-11-18-6-2-1-3-13(18)9-16-20/h1-8H,9-11H2,(H-,15,19)/q+1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025133

(2-(4-Chloro-phenyl)-N-hydroxy-2-oxo-thioacetimidic...)Show InChI InChI=1S/C14H19ClN2O2S/c1-3-17(4-2)9-10-20-14(16-19)13(18)11-5-7-12(15)8-6-11/h5-8,14H,3-4,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025130

(2-(4-Bromo-phenyl)-N-hydroxy-2-oxo-thioacetimidic ...)Show InChI InChI=1S/C12H15BrN2O2S/c1-15(2)7-8-18-12(14-17)11(16)9-3-5-10(13)6-4-9/h3-6,12H,7-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025127
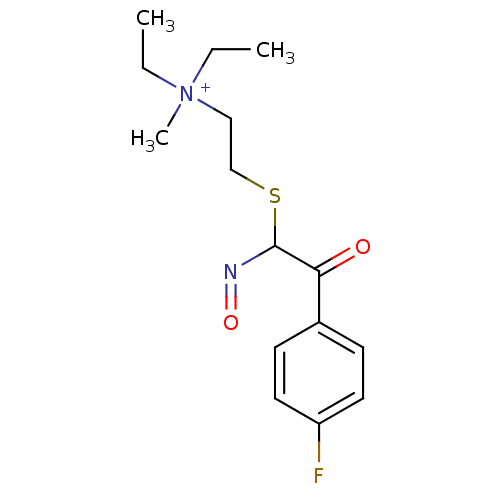
(CHEMBL416644 | Diethyl-{2-[2-(4-fluoro-phenyl)-N-h...)Show InChI InChI=1S/C15H22FN2O2S/c1-4-18(3,5-2)10-11-21-15(17-20)14(19)12-6-8-13(16)9-7-12/h6-9,15H,4-5,10-11H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025143
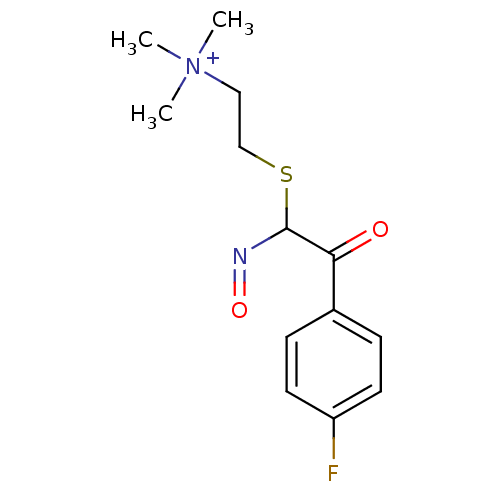
(CHEMBL297316 | {2-[2-(4-Fluoro-phenyl)-N-hydroxy-2...)Show InChI InChI=1S/C13H18FN2O2S/c1-16(2,3)8-9-19-13(15-18)12(17)10-4-6-11(14)7-5-10/h4-7,13H,8-9H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025145
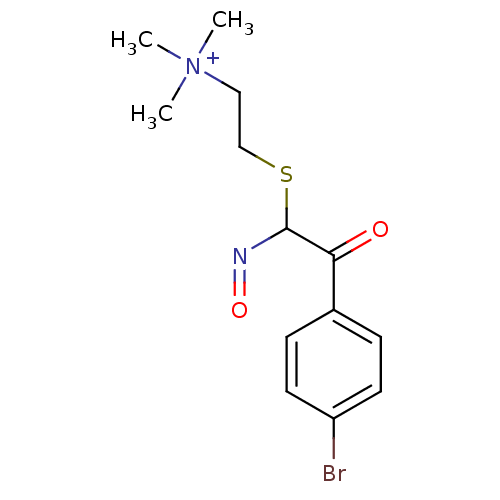
(CHEMBL296560 | {2-[2-(4-Bromo-phenyl)-N-hydroxy-2-...)Show InChI InChI=1S/C13H18BrN2O2S/c1-16(2,3)8-9-19-13(15-18)12(17)10-4-6-11(14)7-5-10/h4-7,13H,8-9H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025144

(CHEMBL47375 | {2-[N-Hydroxy-2-(4-methoxy-phenyl)-2...)Show InChI InChI=1S/C14H21N2O3S/c1-16(2,3)9-10-20-14(15-18)13(17)11-5-7-12(19-4)8-6-11/h5-8,14H,9-10H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025136

(CHEMBL556172 | N-Hydroxy-2-oxo-2-phenyl-thioacetim...)Show InChI InChI=1S/C14H20N2O2S/c1-3-16(4-2)10-11-19-14(15-18)13(17)12-8-6-5-7-9-12/h5-9,14H,3-4,10-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025135
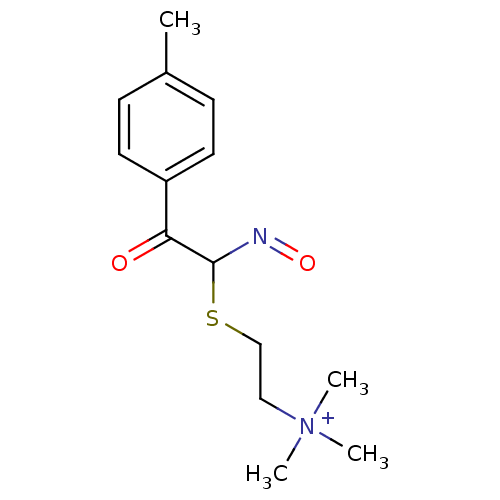
(CHEMBL296429 | [2-(N-Hydroxy-2-oxo-2-p-tolyl-aceti...)Show InChI InChI=1S/C14H21N2O2S/c1-11-5-7-12(8-6-11)13(17)14(15-18)19-10-9-16(2,3)4/h5-8,14H,9-10H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025134

(2-(4-Chloro-phenyl)-N-hydroxy-2-oxo-thioacetimidic...)Show InChI InChI=1S/C12H15ClN2O2S/c1-15(2)7-8-18-12(14-17)11(16)9-3-5-10(13)6-4-9/h3-6,12H,7-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025138
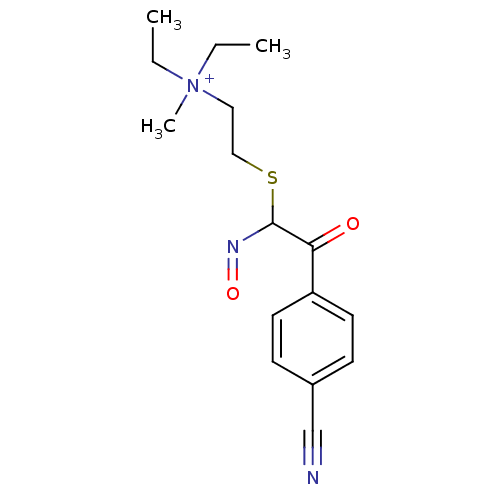
(CHEMBL296277 | {2-[2-(4-Cyano-phenyl)-N-hydroxy-2-...)Show InChI InChI=1S/C16H22N3O2S/c1-4-19(3,5-2)10-11-22-16(18-21)15(20)14-8-6-13(12-17)7-9-14/h6-9,16H,4-5,10-11H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025139
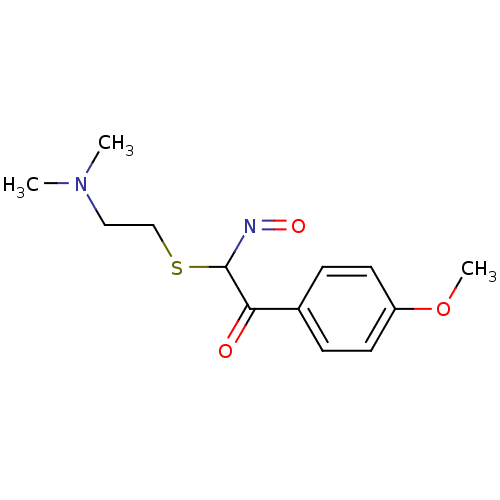
(CHEMBL416464 | N-Hydroxy-2-(4-methoxy-phenyl)-2-ox...)Show InChI InChI=1S/C13H18N2O3S/c1-15(2)8-9-19-13(14-17)12(16)10-4-6-11(18-3)7-5-10/h4-7,13H,8-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025132
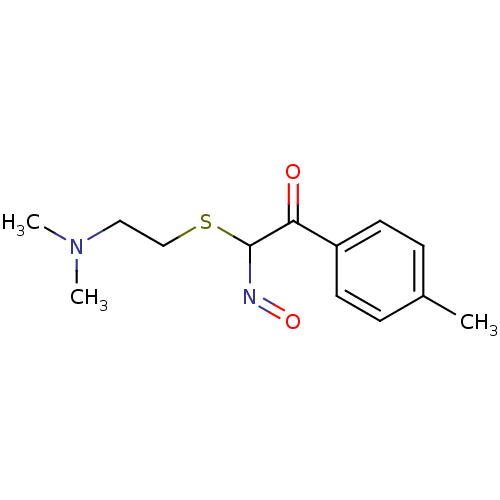
(CHEMBL542609 | N-Hydroxy-2-oxo-2-p-tolyl-thioaceti...)Show InChI InChI=1S/C13H18N2O2S/c1-10-4-6-11(7-5-10)12(16)13(14-17)18-9-8-15(2)3/h4-7,13H,8-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025128

(CHEMBL296786 | {2-[2-(4-Chloro-phenyl)-N-hydroxy-2...)Show InChI InChI=1S/C15H22ClN2O2S/c1-4-18(3,5-2)10-11-21-15(17-20)14(19)12-6-8-13(16)9-7-12/h6-9,15H,4-5,10-11H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of Human AchE |
J Med Chem 29: 1689-96 (1986)
BindingDB Entry DOI: 10.7270/Q2TH8N8Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data