

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine racemase (Homo sapiens (Human)) | BDBM50055467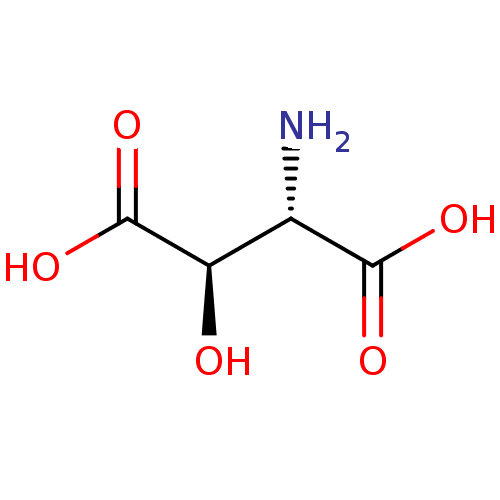 (CHEBI:17576 | CHEMBL3317781) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Binding affinity to serine recemase (unknown origin) | Bioorg Med Chem Lett 24: 3732-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.003 BindingDB Entry DOI: 10.7270/Q2PR7XNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine racemase (Homo sapiens (Human)) | BDBM14673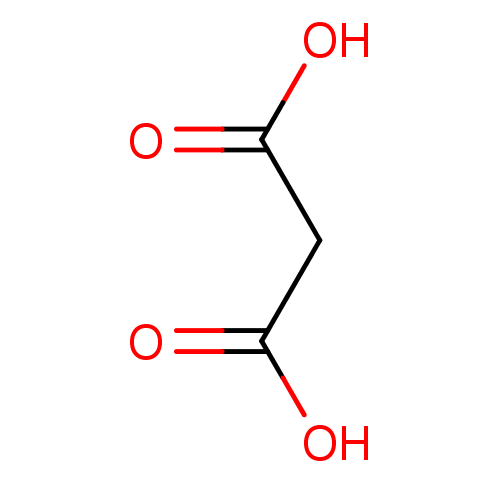 (Fragment 3 | Malonic Acid | propanedioic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Binding affinity to serine recemase (unknown origin) | Bioorg Med Chem Lett 24: 3732-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.003 BindingDB Entry DOI: 10.7270/Q2PR7XNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine racemase (Homo sapiens (Human)) | BDBM18133 (2-aminoacetic acid | CHEMBL773 | Glycine | [14C]Gl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem | Article PubMed | 3.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Binding affinity to serine recemase (unknown origin) | Bioorg Med Chem Lett 24: 3732-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.003 BindingDB Entry DOI: 10.7270/Q2PR7XNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50152125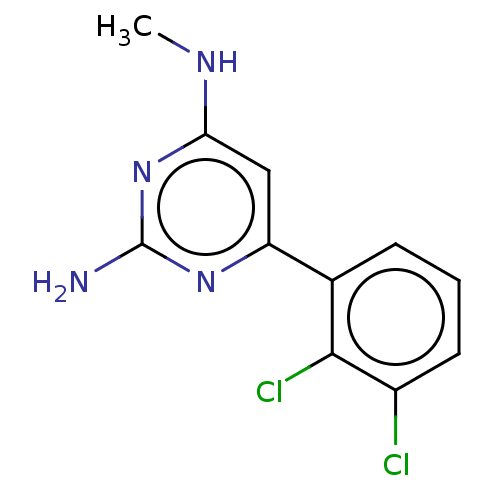 (CHEMBL3781316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal 6xHis-tagged MTH1 (3 to 156 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using dGTP ... | Eur J Med Chem 167: 153-160 (2019) Article DOI: 10.1016/j.ejmech.2019.02.011 BindingDB Entry DOI: 10.7270/Q20005CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50509698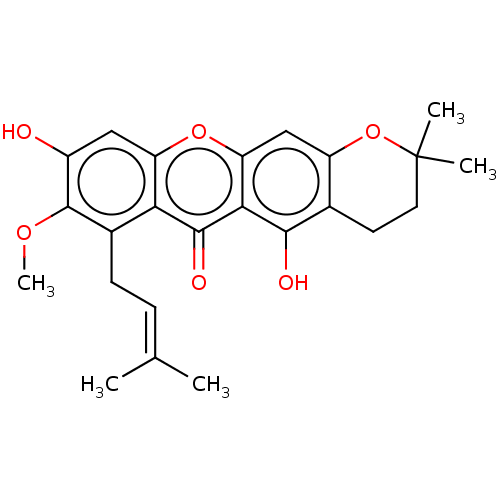 (3-Isomangostin | CHEMBL464119) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal 6xHis-tagged MTH1 (3 to 156 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using dGTP ... | Eur J Med Chem 167: 153-160 (2019) Article DOI: 10.1016/j.ejmech.2019.02.011 BindingDB Entry DOI: 10.7270/Q20005CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50214969 (1,3,6-Trihydroxy-7-methoxy-2,8-bis-(3-methyl-but-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal 6xHis-tagged MTH1 (3 to 156 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using dGTP ... | Eur J Med Chem 167: 153-160 (2019) Article DOI: 10.1016/j.ejmech.2019.02.011 BindingDB Entry DOI: 10.7270/Q20005CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Death-associated protein kinase 1 (Homo sapiens (Human)) | BDBM26658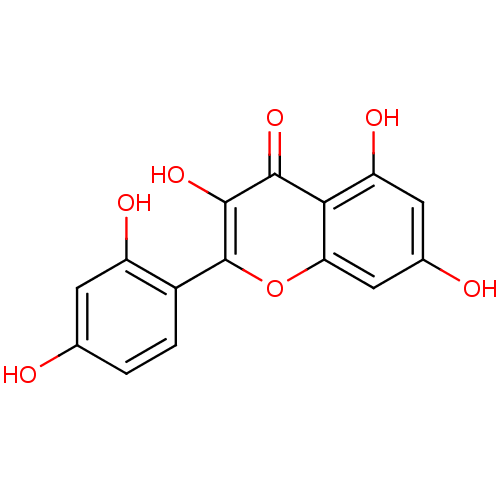 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Displacement of ANS from DAPK1 catalytic domain (1 to 285) (unknown origin) after 30 mins by fluorescence assay | J Med Chem 58: 7400-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00893 BindingDB Entry DOI: 10.7270/Q21838BM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50509700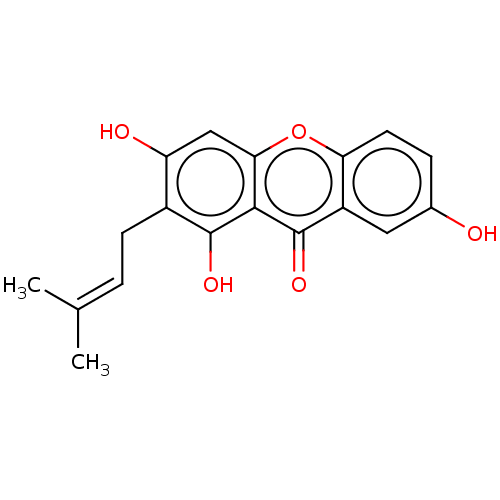 (CHEMBL506229) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal 6xHis-tagged MTH1 (3 to 156 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using dGTP ... | Eur J Med Chem 167: 153-160 (2019) Article DOI: 10.1016/j.ejmech.2019.02.011 BindingDB Entry DOI: 10.7270/Q20005CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50352564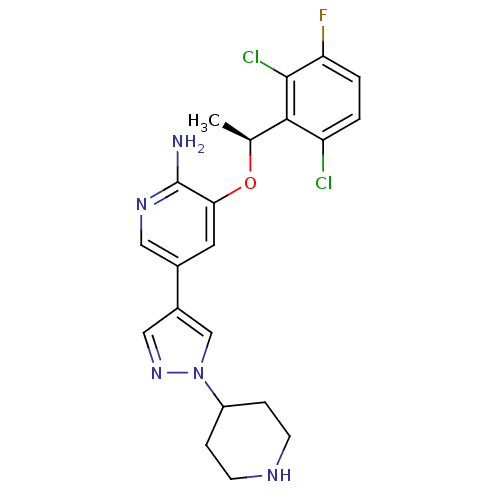 (CHEMBL1825141) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal 6xHis-tagged MTH1 (3 to 156 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using dGTP ... | Eur J Med Chem 167: 153-160 (2019) Article DOI: 10.1016/j.ejmech.2019.02.011 BindingDB Entry DOI: 10.7270/Q20005CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50241453 (1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-enyl)-9...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal 6xHis-tagged MTH1 (3 to 156 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using dGTP ... | Eur J Med Chem 167: 153-160 (2019) Article DOI: 10.1016/j.ejmech.2019.02.011 BindingDB Entry DOI: 10.7270/Q20005CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM36880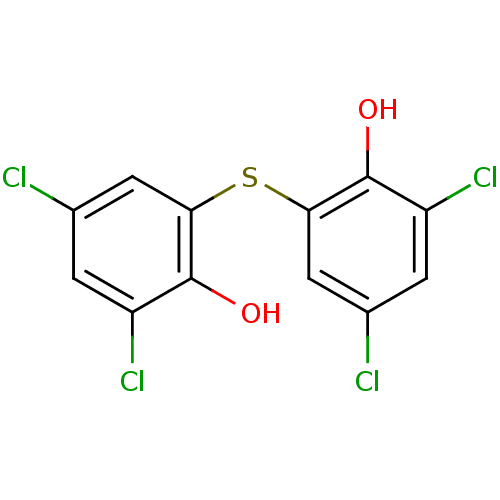 (2,2'-THIOBIS(4,6-DICHLOROPHENOL) | 2,4-dichlor...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Stabilization of TTR V3OM mutant (unknown origin) assessed as acid-mediated protein aggregation inhibition ratio incubated for 1 week by absorbance m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00823 BindingDB Entry DOI: 10.7270/Q2X63RR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50197883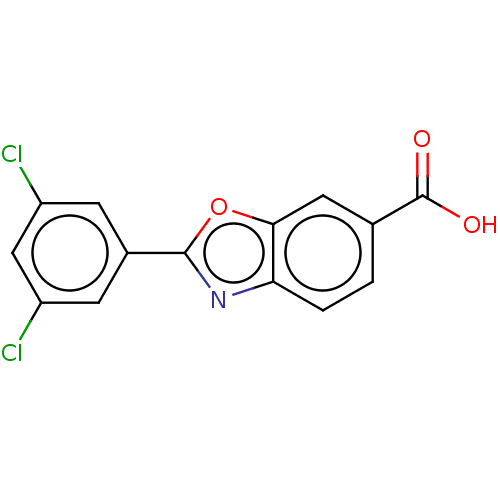 (CHEBI:78538 | FX-1006 | Tafamidis | US10377729, Co...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Stabilization of TTR V3OM mutant (unknown origin) assessed as acid-mediated protein aggregation inhibition ratio incubated for 1 week by absorbance m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00823 BindingDB Entry DOI: 10.7270/Q2X63RR5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50509699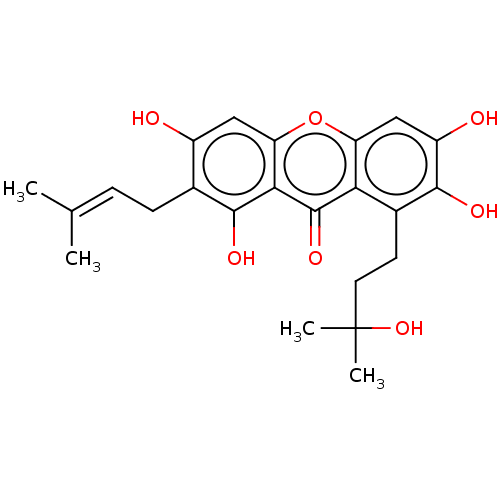 (CHEMBL4440642) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal 6xHis-tagged MTH1 (3 to 156 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using dGTP ... | Eur J Med Chem 167: 153-160 (2019) Article DOI: 10.1016/j.ejmech.2019.02.011 BindingDB Entry DOI: 10.7270/Q20005CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM36880 (2,2'-THIOBIS(4,6-DICHLOROPHENOL) | 2,4-dichlor...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Stabilization of wild type TTR (unknown origin) expressed in Escherichia coli assessed as reduction in methanol-induced aggregation incubated for 60 ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00823 BindingDB Entry DOI: 10.7270/Q2X63RR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50108877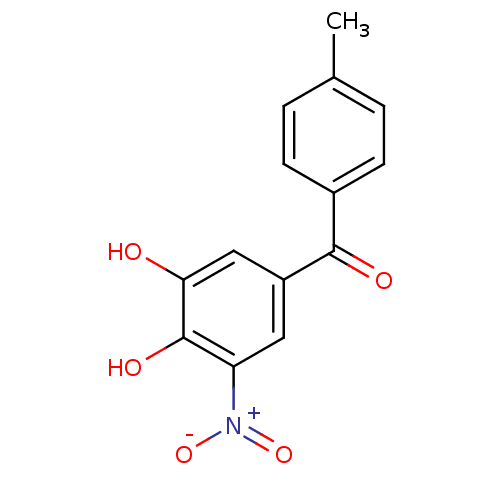 ((3,4-Dihydroxy-5-nitro-phenyl)-p-tolyl-methanone |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Stabilization of TTR V3OM mutant (unknown origin) assessed as acid-mediated protein aggregation inhibition ratio incubated for 1 week by absorbance m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00823 BindingDB Entry DOI: 10.7270/Q2X63RR5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM58491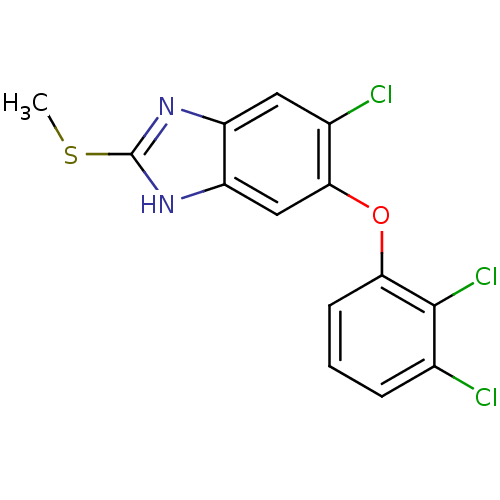 (5-[2,3-bis(chloranyl)phenoxy]-6-chloranyl-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Stabilization of TTR V3OM mutant (unknown origin) assessed as acid-mediated protein aggregation inhibition ratio incubated for 1 week by absorbance m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00823 BindingDB Entry DOI: 10.7270/Q2X63RR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50240510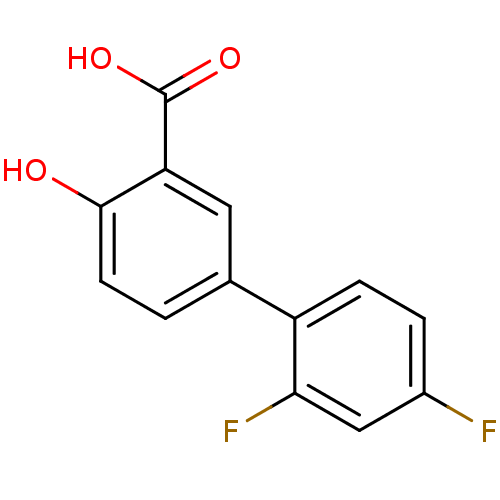 (CHEMBL898 | DIFLUNISAL) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Stabilization of TTR V3OM mutant (unknown origin) assessed as acid-mediated protein aggregation inhibition ratio incubated for 1 week by absorbance m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00823 BindingDB Entry DOI: 10.7270/Q2X63RR5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50197883 (CHEBI:78538 | FX-1006 | Tafamidis | US10377729, Co...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM32018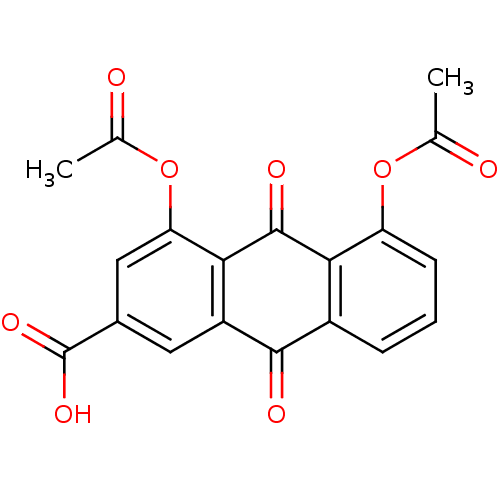 (1,8-DIACETOXY-3-CARBOXYANTHRAQUINONE | 4,5-diaceto...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568451 (CHEMBL4850762) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50240510 (CHEMBL898 | DIFLUNISAL) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568449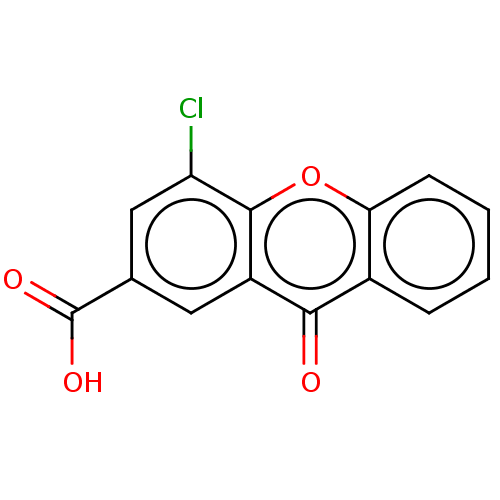 (CHEMBL4851268) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568444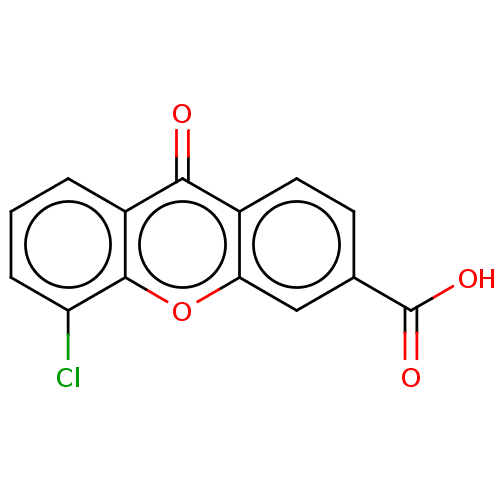 (CHEMBL4866842) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 1 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Displacement of ANS from DAPK1 catalytic domain (1 to 285) (unknown origin) after 30 mins by fluorescence assay | J Med Chem 58: 7400-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00893 BindingDB Entry DOI: 10.7270/Q21838BM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568450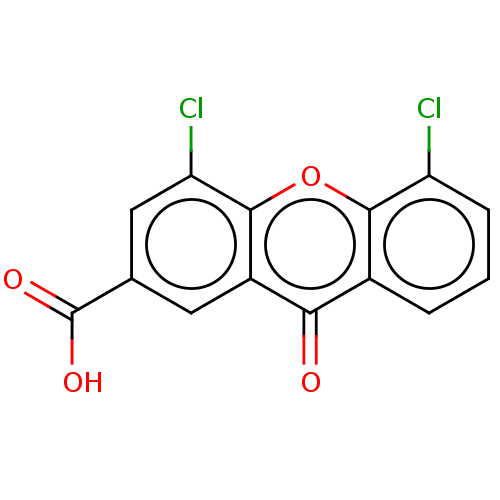 (CHEMBL4855105) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568429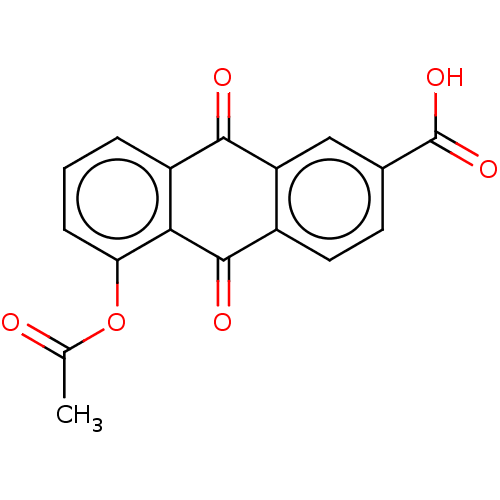 (CHEMBL4860573) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568442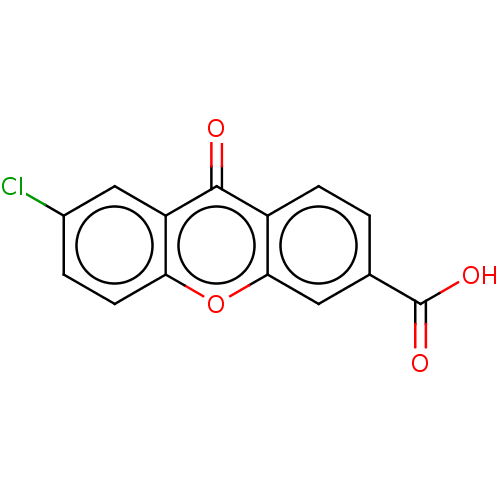 (CHEMBL4859052) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50530238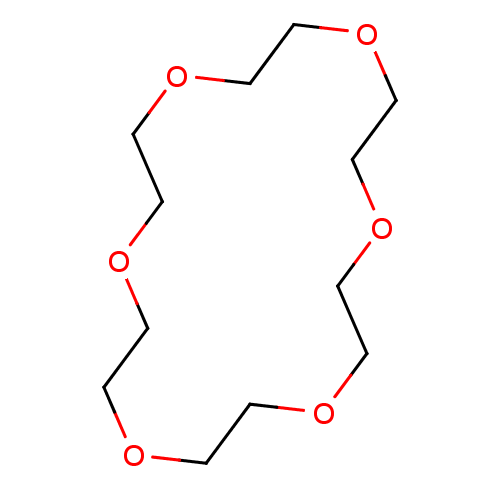 (1,4,7,10,13,16-Hexaoxacyclooctadecane | 1,4,7,10,1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of ANS binding to TTR V30M mutant (unknown origin) expressed in Escherichia coli assessed as BC50 for ANS binding to TTR at 5 uM in presen... | J Med Chem 62: 2076-2082 (2019) Article DOI: 10.1021/acs.jmedchem.8b01700 BindingDB Entry DOI: 10.7270/Q2FB56DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50241453 (1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-enyl)-9...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Death-associated protein kinase 1 (Homo sapiens (Human)) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Displacement of ANS from DAPK1 catalytic domain (1 to 285) (unknown origin) after 30 mins by fluorescence assay | J Med Chem 58: 7400-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00893 BindingDB Entry DOI: 10.7270/Q21838BM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50530238 (1,4,7,10,13,16-Hexaoxacyclooctadecane | 1,4,7,10,1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of ANS binding to TTR V30M mutant (unknown origin) expressed in Escherichia coli assessed as BC50 for ANS binding to TTR at 5 uM in presen... | J Med Chem 62: 2076-2082 (2019) Article DOI: 10.1021/acs.jmedchem.8b01700 BindingDB Entry DOI: 10.7270/Q2FB56DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568441 (CHEMBL4857993) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568425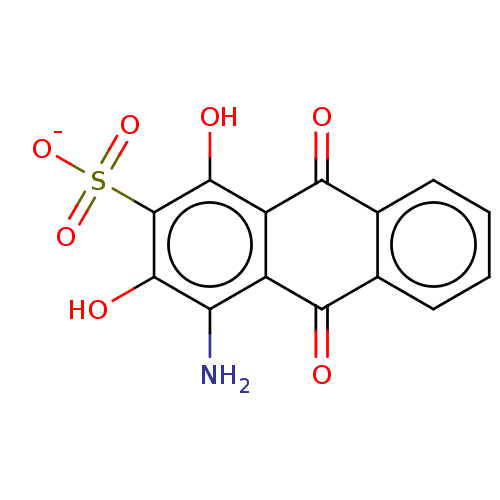 (CHEMBL4870376) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Death-associated protein kinase 1 (Homo sapiens (Human)) | BDBM26658 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of DAPK1 (unknown origin) using ZIPtide as substrate by fluorescence assay in presence of ATP, MgCl2 | J Med Chem 58: 7400-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00893 BindingDB Entry DOI: 10.7270/Q21838BM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568434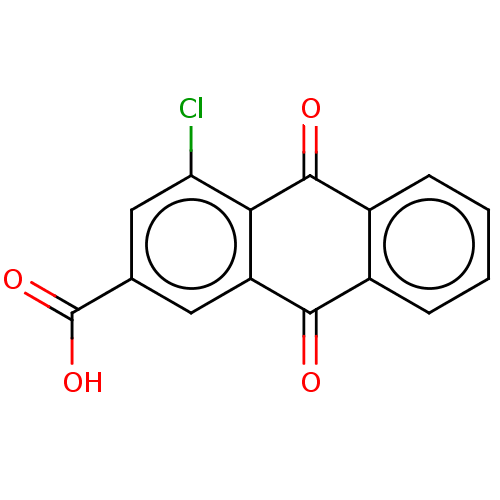 (CHEMBL4849950) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568447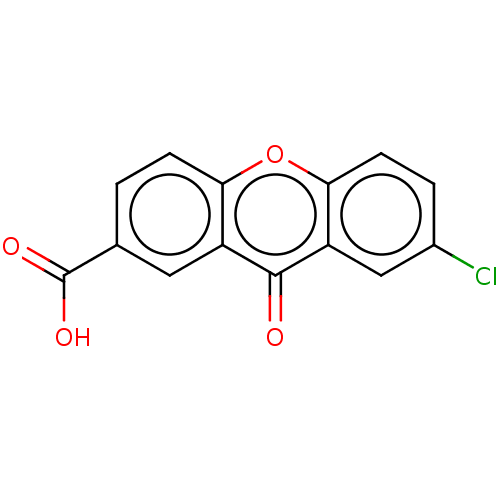 (CHEMBL4874413) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM32021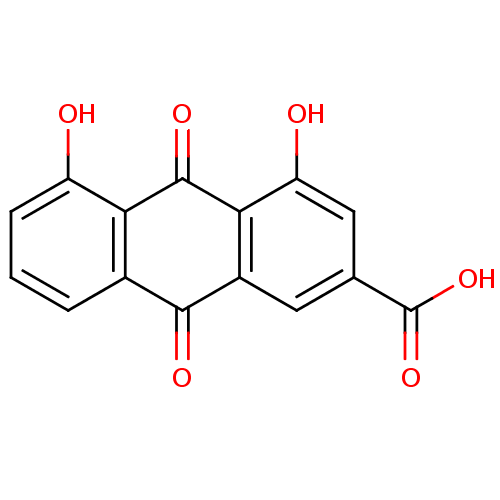 (4,5-bis(oxidanyl)-9,10-bis(oxidanylidene)anthracen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568427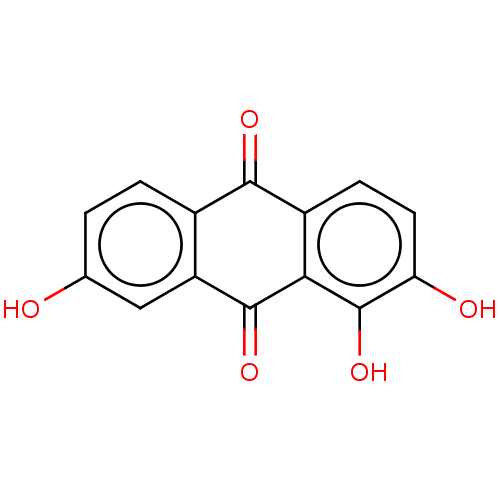 (CHEMBL4864788) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50240510 (CHEMBL898 | DIFLUNISAL) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of ANS binding to TTR V30M mutant (unknown origin) expressed in Escherichia coli assessed as BC50 for ANS binding to TTR at 5 uM in presen... | J Med Chem 62: 2076-2082 (2019) Article DOI: 10.1021/acs.jmedchem.8b01700 BindingDB Entry DOI: 10.7270/Q2FB56DV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50240510 (CHEMBL898 | DIFLUNISAL) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of ANS binding to TTR V30M mutant (unknown origin) expressed in Escherichia coli assessed as BC50 for ANS binding to TTR at 5 uM in presen... | J Med Chem 62: 2076-2082 (2019) Article DOI: 10.1021/acs.jmedchem.8b01700 BindingDB Entry DOI: 10.7270/Q2FB56DV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM67454 (1,2,4-trihydroxy-9,10-anthraquinone | 1,2,4-trihyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568432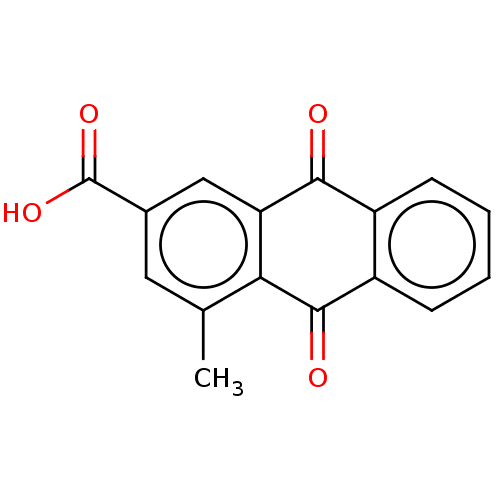 (CHEMBL4864022) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568448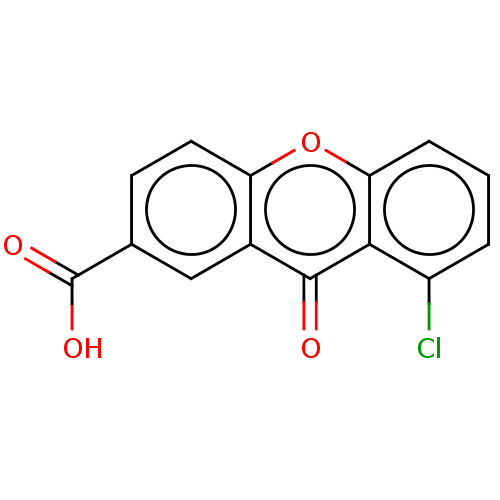 (CHEMBL4875739) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568443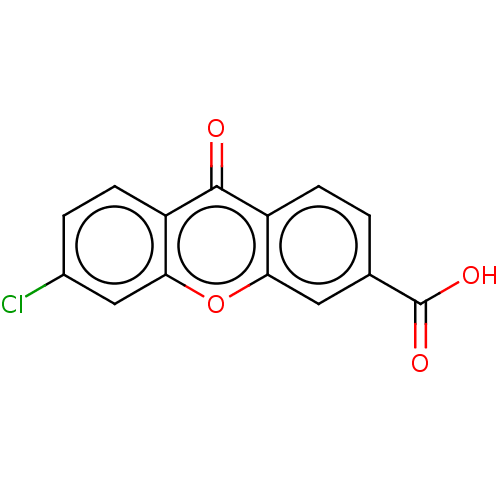 (CHEMBL4866802) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568445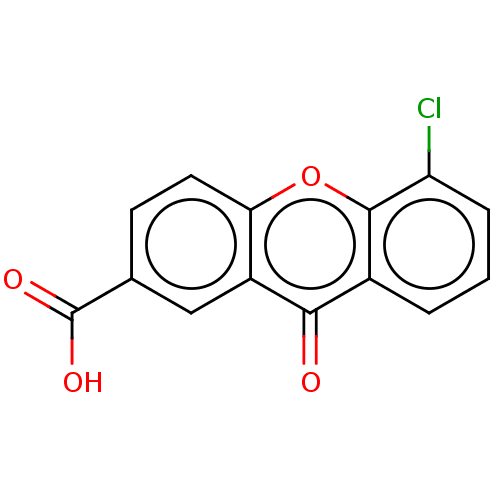 (CHEMBL4868175) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568440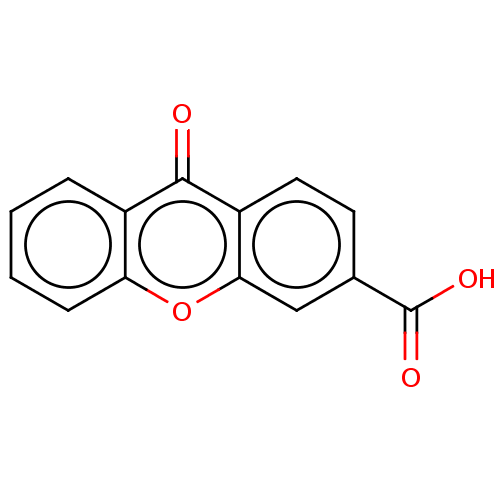 (CHEMBL4861683) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50311742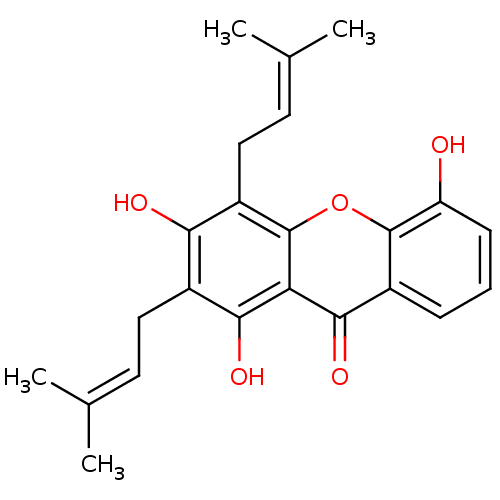 (8-DEOXYGARTANIN | 8-desoxygartanin | CHEMBL488606) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal 6xHis-tagged MTH1 (3 to 156 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using dGTP ... | Eur J Med Chem 167: 153-160 (2019) Article DOI: 10.1016/j.ejmech.2019.02.011 BindingDB Entry DOI: 10.7270/Q20005CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50530238 (1,4,7,10,13,16-Hexaoxacyclooctadecane | 1,4,7,10,1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of ANS binding to TTR V30M mutant (unknown origin) expressed in Escherichia coli assessed as BC50 for ANS binding to TTR at 100 mM in pres... | J Med Chem 62: 2076-2082 (2019) Article DOI: 10.1021/acs.jmedchem.8b01700 BindingDB Entry DOI: 10.7270/Q2FB56DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50530238 (1,4,7,10,13,16-Hexaoxacyclooctadecane | 1,4,7,10,1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of ANS binding to TTR V30M mutant (unknown origin) expressed in Escherichia coli assessed as BC50 for ANS binding to TTR at 100 mM in pres... | J Med Chem 62: 2076-2082 (2019) Article DOI: 10.1021/acs.jmedchem.8b01700 BindingDB Entry DOI: 10.7270/Q2FB56DV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50568433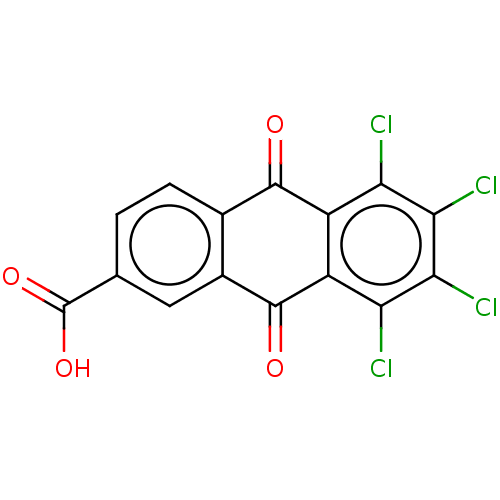 (CHEMBL4876565) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of acid-mediated aggregation of TTR V30M mutant (unknown origin) expressed in Escherichia coli pretreated for 30 mins at pH 7 followed by ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116292 BindingDB Entry DOI: 10.7270/Q2222ZH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 130 total ) | Next | Last >> |