

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50275270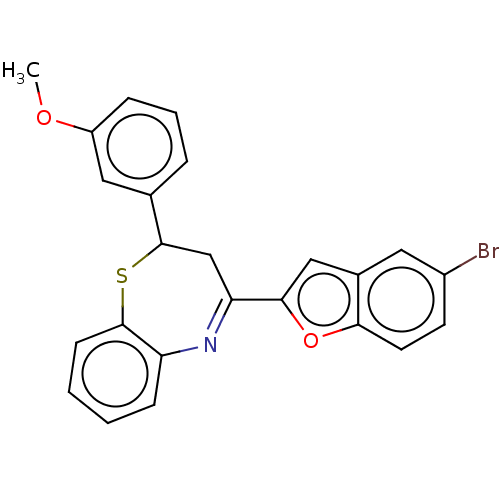 (CHEMBL4126207) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BChE using varying levels of butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrat... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131762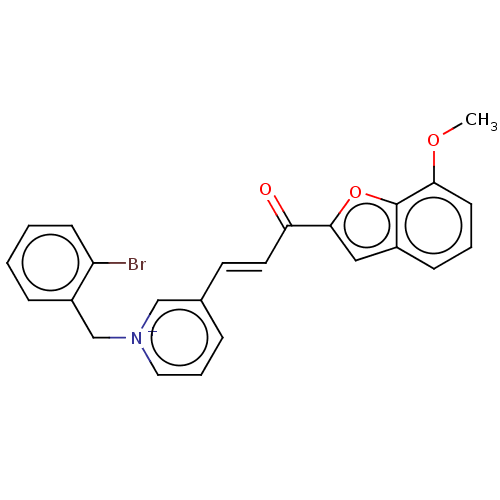 (CHEMBL3633865) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131771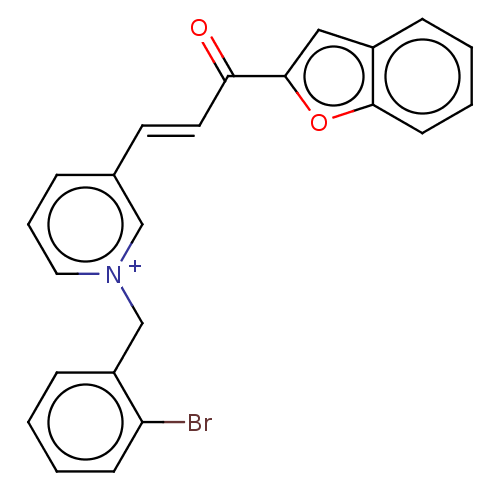 (CHEMBL3633525) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131718 (CHEMBL3633870) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131772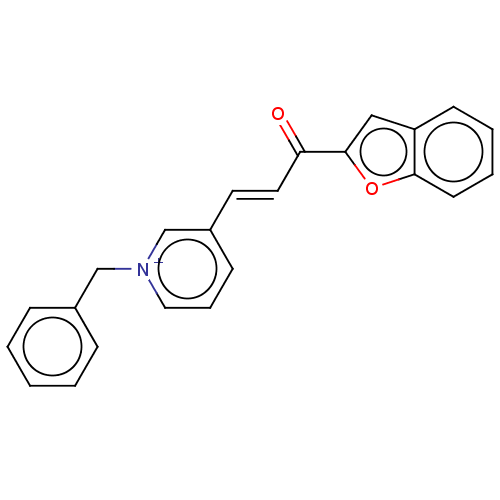 (CHEMBL3633524) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131758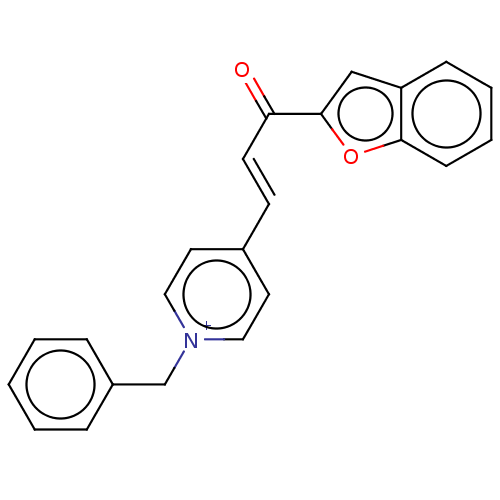 (CHEMBL3633869) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131770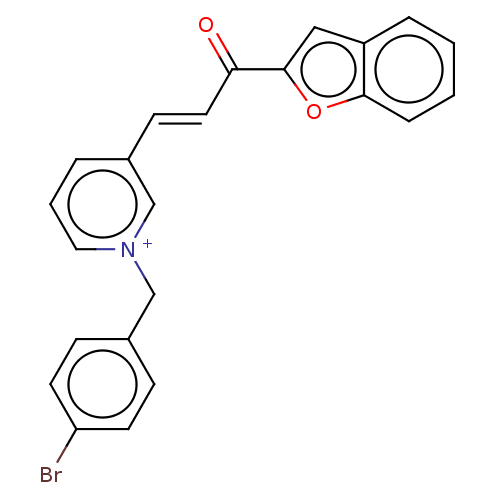 (CHEMBL3633857) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 357 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131764 (CHEMBL3633863) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 786 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131759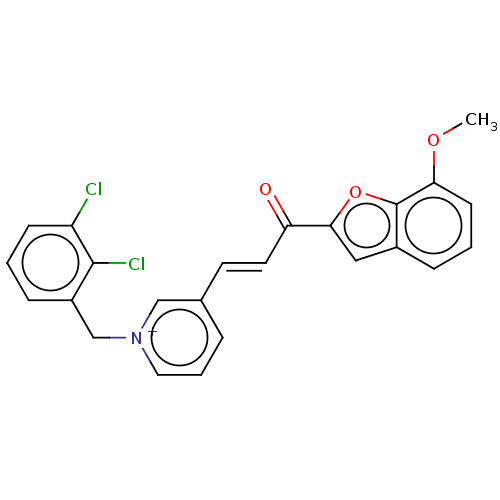 (CHEMBL3633868) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 985 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275270 (CHEMBL4126207) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275230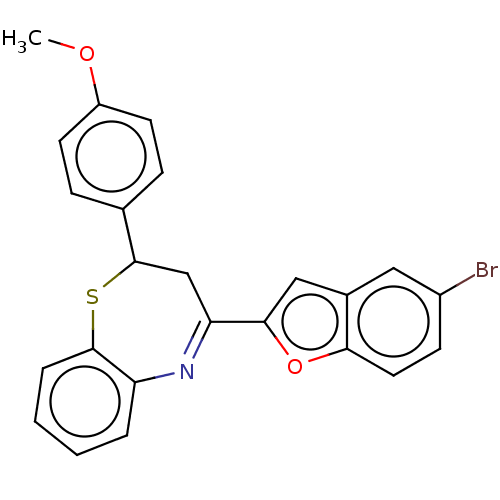 (CHEMBL4126049) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275269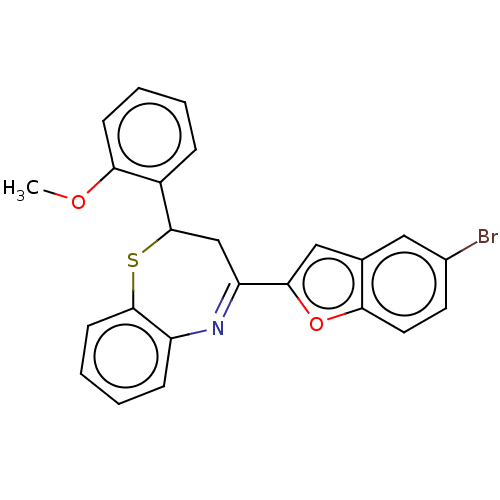 (CHEMBL4129504) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131767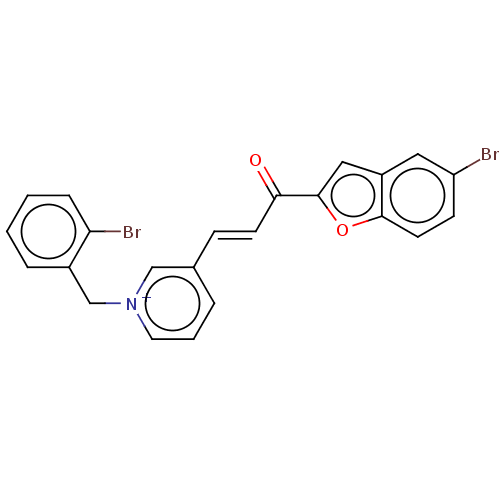 (CHEMBL3633860) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275253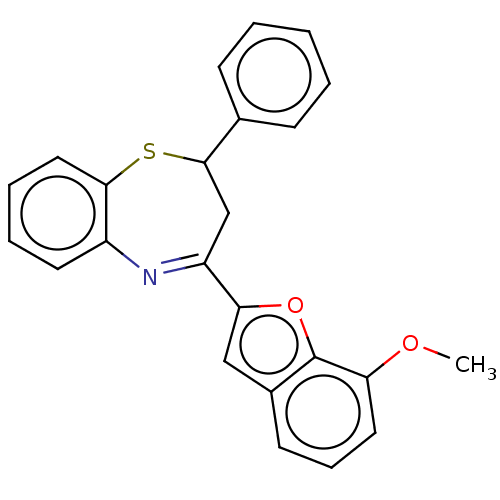 (CHEMBL4130033) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131765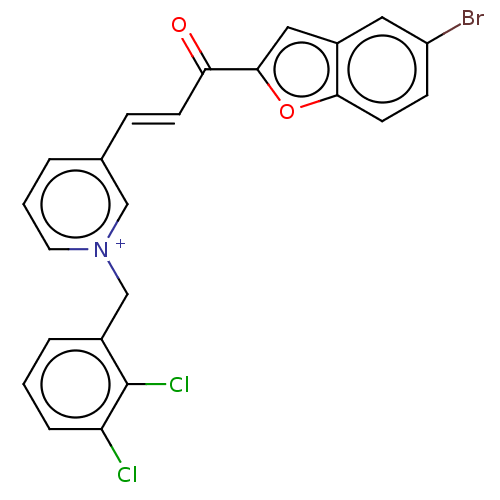 (CHEMBL3633862) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131717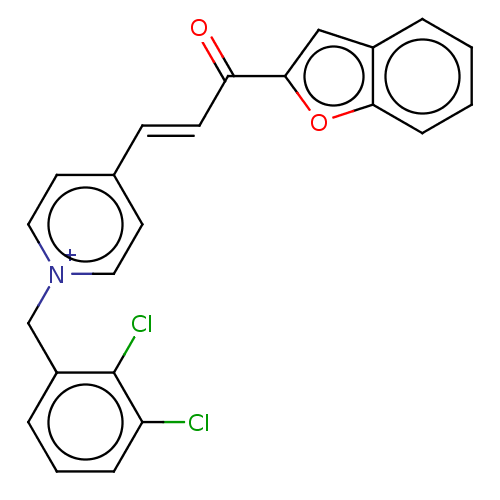 (CHEMBL3633871) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131763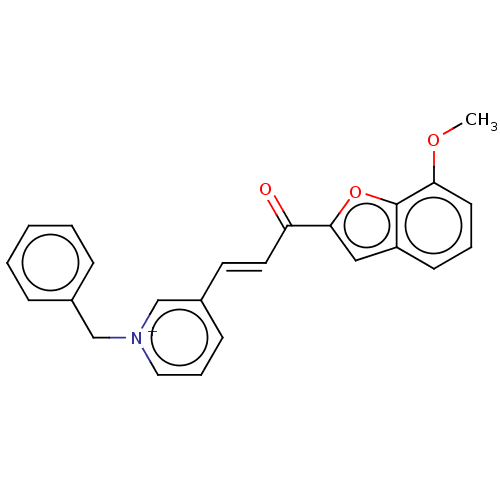 (CHEMBL3633864) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275245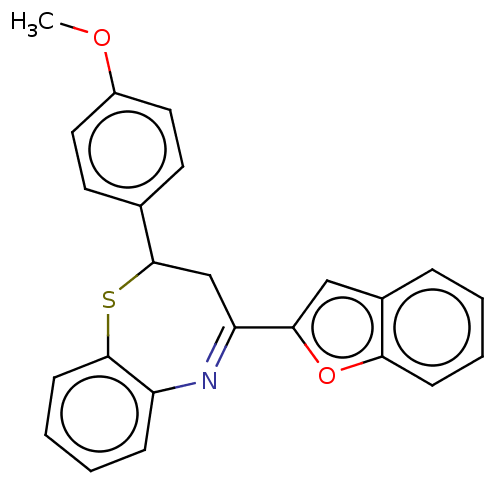 (CHEMBL4126460) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275244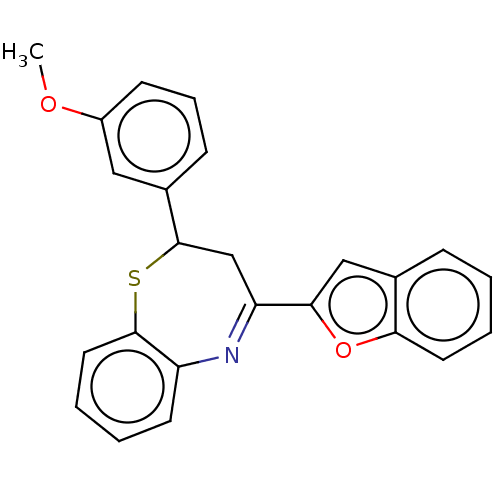 (CHEMBL4127850) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275254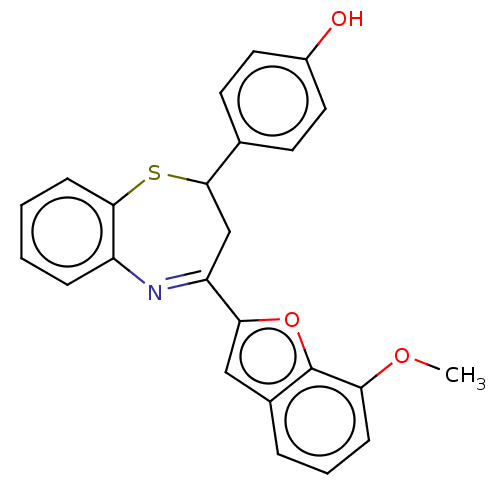 (CHEMBL4128663) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275238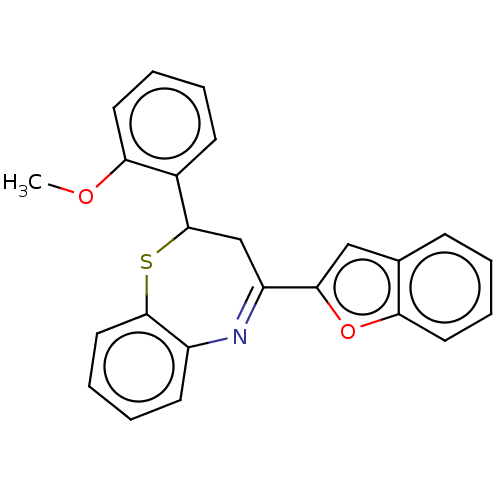 (CHEMBL4130145) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131768 (CHEMBL3633859) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131715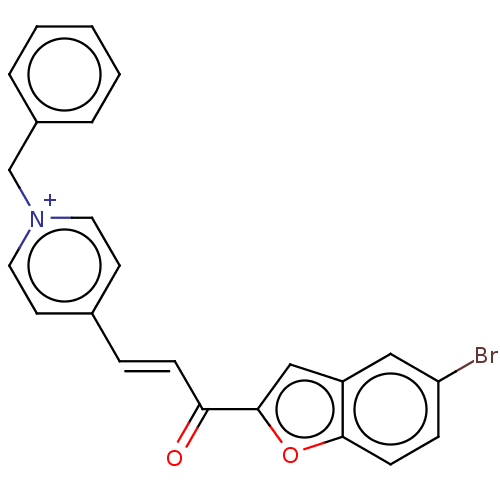 (CHEMBL3633872) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275268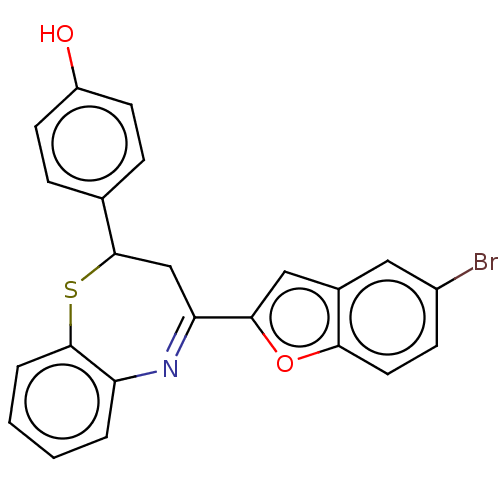 (CHEMBL4129645) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275255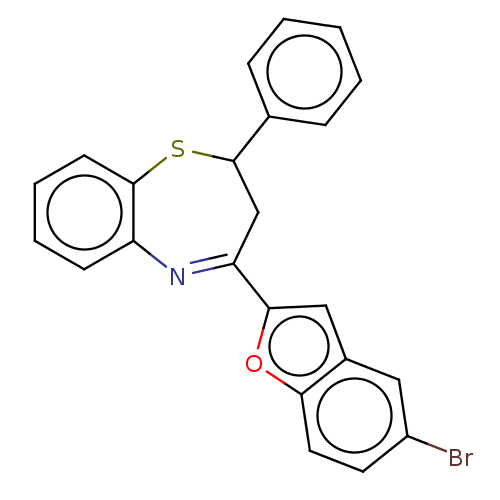 (CHEMBL4128273) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275271 (CHEMBL4127431) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131761 (CHEMBL3633866) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131760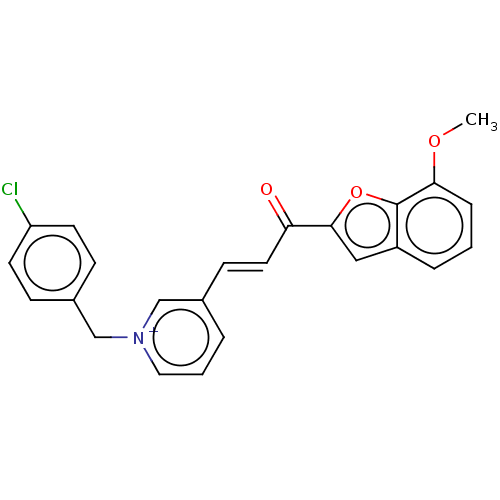 (CHEMBL3633867) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275272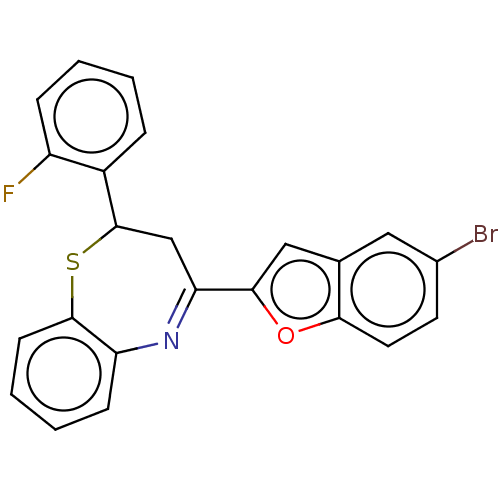 (CHEMBL4127288) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275237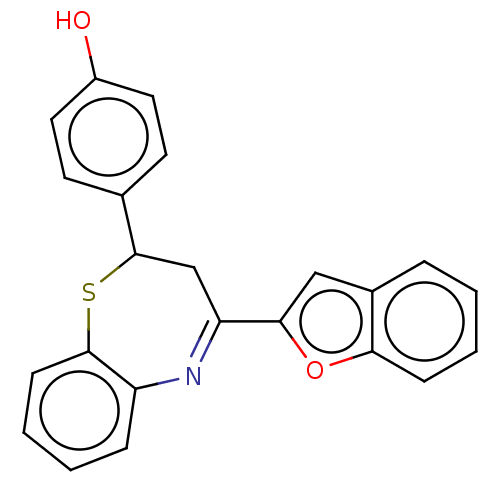 (CHEMBL4126881) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275309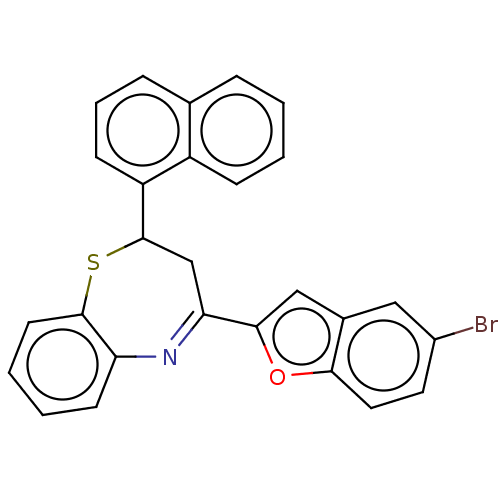 (CHEMBL4128803) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275310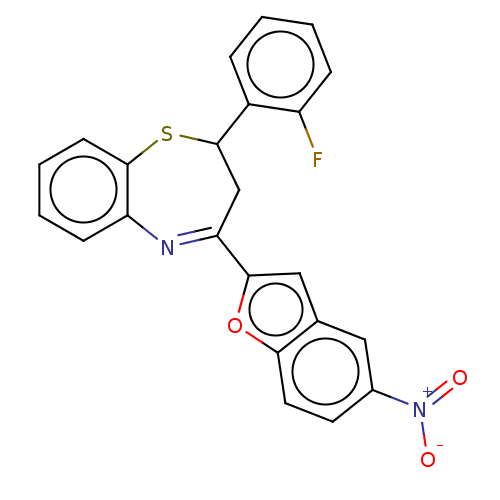 (CHEMBL4129157) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275231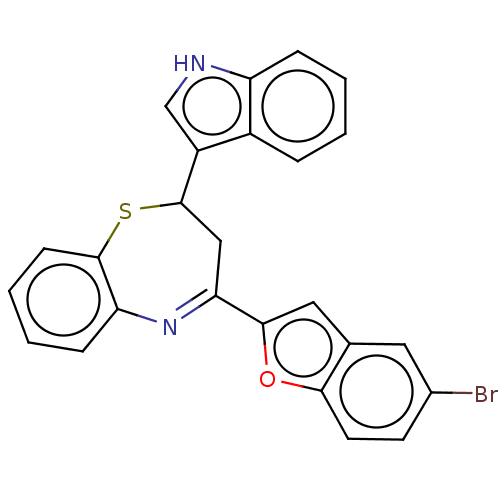 (CHEMBL4127739) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131769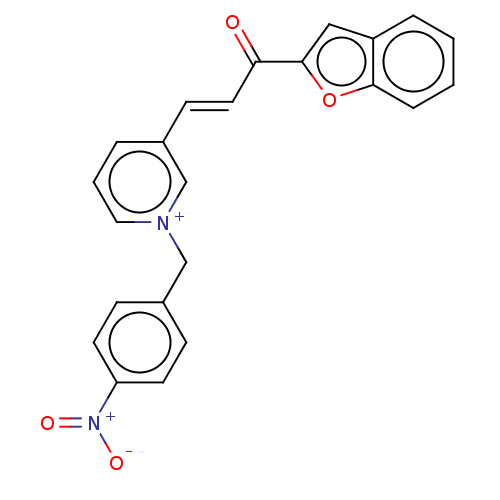 (CHEMBL3633858) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50131766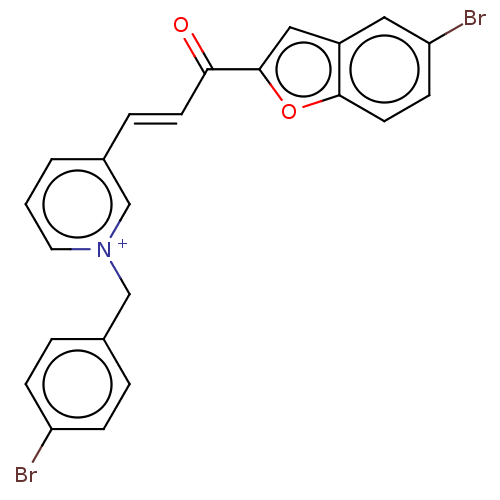 (CHEMBL3633861) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE (type VI-S) using acetylthiocholine iodide as substrate assessed as 5-thio-2-nitrobenzoate anion formation pre... | Eur J Med Chem 103: 361-9 (2015) Article DOI: 10.1016/j.ejmech.2015.08.061 BindingDB Entry DOI: 10.7270/Q2XK8HC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275311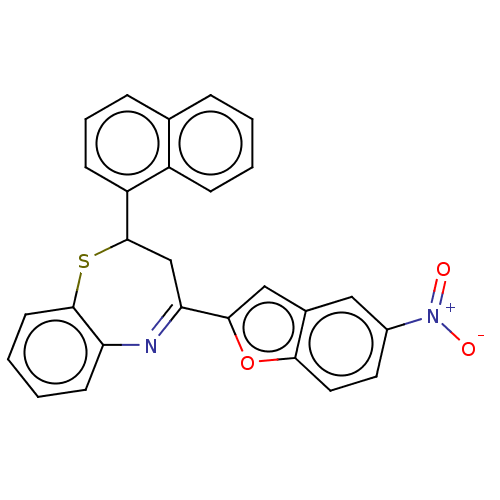 (CHEMBL4129309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275247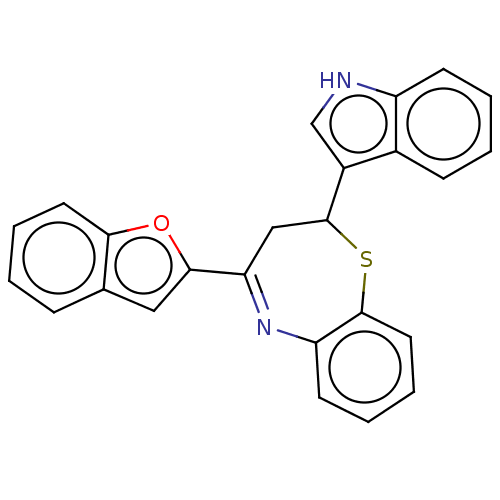 (CHEMBL4127693) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275246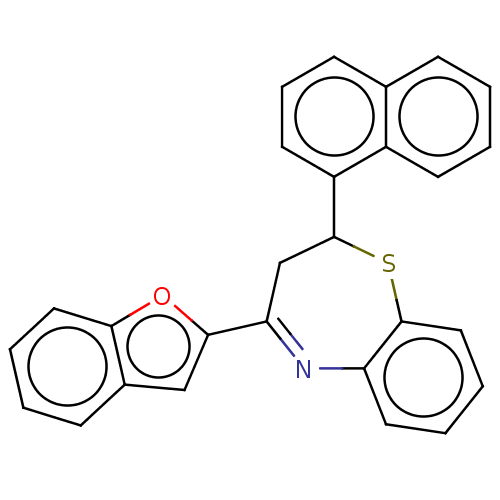 (CHEMBL4129086) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50275236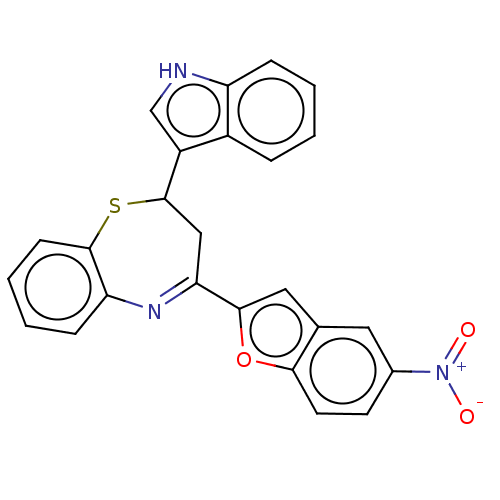 (CHEMBL4127914) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alzahra University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate pretreated for 15 mins followed by substrate addition measured for 6 min... | Bioorg Med Chem 26: 3076-3095 (2018) Article DOI: 10.1016/j.bmc.2018.02.049 BindingDB Entry DOI: 10.7270/Q2SF2ZPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||