 Found 117 hits with Last Name = 'moy' and Initial = 'e'
Found 117 hits with Last Name = 'moy' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0269 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.545 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.679 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 32.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 62.3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.346 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159756

(PF-02384554 | US10966980, Example 8 | US9035074, 8)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1cc(ccn1)C#N)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-5.61,1.61,;-4.28,.84,;-2.94,1.61,;-2.55,3.09,;-1.06,2.69,;-1.46,1.21,;.28,3.47,;1.61,2.69,;.52,1.61,;2.7,3.78,;2.94,1.93,;2.94,.38,;4.28,-.38,;5.61,.38,;5.61,1.93,;4.28,2.69,;4.28,-1.92,;4.28,-3.46,;-4.28,-.7,;-5.61,-1.47,;-5.61,-3.01,;-4.28,-3.78,;-2.94,-3.01,;-1.48,-3.49,;-.57,-2.24,;-1.48,-1,;-2.94,-1.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM159756

(PF-02384554 | US10966980, Example 8 | US9035074, 8)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1cc(ccn1)C#N)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-5.61,1.61,;-4.28,.84,;-2.94,1.61,;-2.55,3.09,;-1.06,2.69,;-1.46,1.21,;.28,3.47,;1.61,2.69,;.52,1.61,;2.7,3.78,;2.94,1.93,;2.94,.38,;4.28,-.38,;5.61,.38,;5.61,1.93,;4.28,2.69,;4.28,-1.92,;4.28,-3.46,;-4.28,-.7,;-5.61,-1.47,;-5.61,-3.01,;-4.28,-3.78,;-2.94,-3.01,;-1.48,-3.49,;-.57,-2.24,;-1.48,-1,;-2.94,-1.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50463770
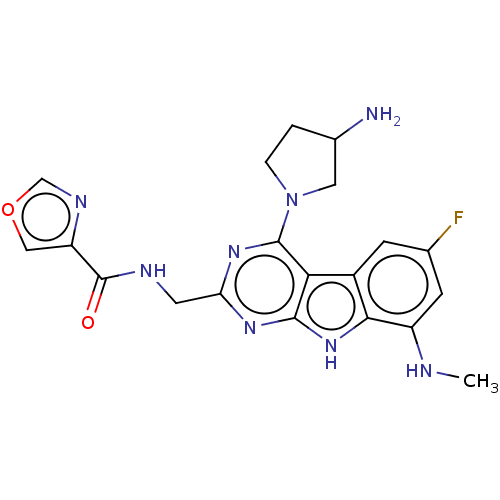
(CHEMBL4238919)Show SMILES CNc1cc(F)cc2c1[nH]c1nc(CNC(=O)c3cocn3)nc(N3CCC(N)C3)c21 Show InChI InChI=1S/C20H21FN8O2/c1-23-13-5-10(21)4-12-16-18(28-17(12)13)26-15(6-24-20(30)14-8-31-9-25-14)27-19(16)29-3-2-11(22)7-29/h4-5,8-9,11,23H,2-3,6-7,22H2,1H3,(H,24,30)(H,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Redx Pharma
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DNA gyrase activity |
Bioorg Med Chem Lett 28: 2998-3003 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.049
BindingDB Entry DOI: 10.7270/Q2G73HD3 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50463771

(CHEMBL4250651)Show SMILES CNc1cc(F)cc2c1[nH]c1nc(CNC(=O)c3cnco3)nc(N3CCC(N)C3)c21 Show InChI InChI=1S/C20H21FN8O2/c1-23-13-5-10(21)4-12-16-18(28-17(12)13)26-15(7-25-20(30)14-6-24-9-31-14)27-19(16)29-3-2-11(22)8-29/h4-6,9,11,23H,2-3,7-8,22H2,1H3,(H,25,30)(H,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Redx Pharma
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DNA gyrase activity |
Bioorg Med Chem Lett 28: 2998-3003 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.049
BindingDB Entry DOI: 10.7270/Q2G73HD3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50226181
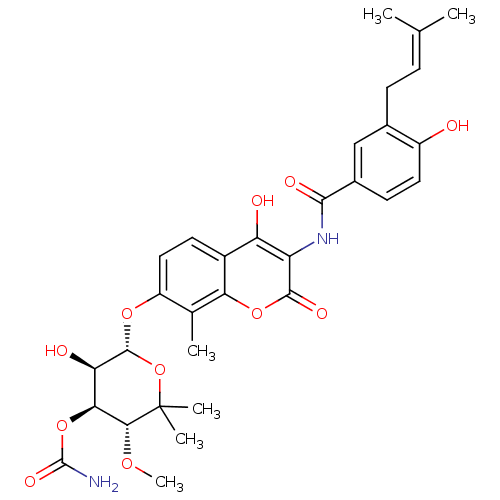
((3R,4S,5R,6R)-5-hydroxy-6-(4-hydroxy-3-(4-hydroxy-...)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@@H](-[#8]-[#6](-[#7])=O)-[#6@@H](-[#8])-[#6@H](-[#8]-c2ccc3c(-[#8])c(-[#7]-[#6](=O)-c4ccc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6])c4)c(=O)oc3c2-[#6])-[#8]C1([#6])[#6] |r| Show InChI InChI=1S/C31H36N2O11/c1-14(2)7-8-16-13-17(9-11-19(16)34)27(37)33-21-22(35)18-10-12-20(15(3)24(18)42-28(21)38)41-29-23(36)25(43-30(32)39)26(40-6)31(4,5)44-29/h7,9-13,23,25-26,29,34-36H,8H2,1-6H3,(H2,32,39)(H,33,37)/t23-,25+,26-,29-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Redx Pharma
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DNA gyrase ATPase activity |
Bioorg Med Chem Lett 28: 2998-3003 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.049
BindingDB Entry DOI: 10.7270/Q2G73HD3 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50463770

(CHEMBL4238919)Show SMILES CNc1cc(F)cc2c1[nH]c1nc(CNC(=O)c3cocn3)nc(N3CCC(N)C3)c21 Show InChI InChI=1S/C20H21FN8O2/c1-23-13-5-10(21)4-12-16-18(28-17(12)13)26-15(6-24-20(30)14-8-31-9-25-14)27-19(16)29-3-2-11(22)7-29/h4-5,8-9,11,23H,2-3,6-7,22H2,1H3,(H,24,30)(H,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Redx Pharma
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DNA gyrase ATPase activity |
Bioorg Med Chem Lett 28: 2998-3003 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.049
BindingDB Entry DOI: 10.7270/Q2G73HD3 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600005
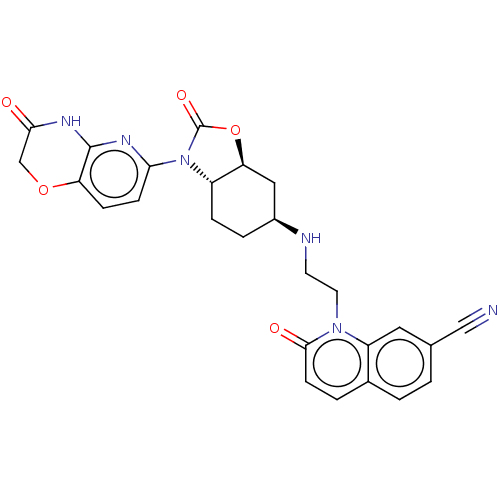
(CHEMBL5195268)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2cc(ccc2ccc1=O)C#N |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33.1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM159756

(PF-02384554 | US10966980, Example 8 | US9035074, 8)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1cc(ccn1)C#N)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-5.61,1.61,;-4.28,.84,;-2.94,1.61,;-2.55,3.09,;-1.06,2.69,;-1.46,1.21,;.28,3.47,;1.61,2.69,;.52,1.61,;2.7,3.78,;2.94,1.93,;2.94,.38,;4.28,-.38,;5.61,.38,;5.61,1.93,;4.28,2.69,;4.28,-1.92,;4.28,-3.46,;-4.28,-.7,;-5.61,-1.47,;-5.61,-3.01,;-4.28,-3.78,;-2.94,-3.01,;-1.48,-3.49,;-.57,-2.24,;-1.48,-1,;-2.94,-1.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM159756

(PF-02384554 | US10966980, Example 8 | US9035074, 8)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1cc(ccn1)C#N)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-5.61,1.61,;-4.28,.84,;-2.94,1.61,;-2.55,3.09,;-1.06,2.69,;-1.46,1.21,;.28,3.47,;1.61,2.69,;.52,1.61,;2.7,3.78,;2.94,1.93,;2.94,.38,;4.28,-.38,;5.61,.38,;5.61,1.93,;4.28,2.69,;4.28,-1.92,;4.28,-3.46,;-4.28,-.7,;-5.61,-1.47,;-5.61,-3.01,;-4.28,-3.78,;-2.94,-3.01,;-1.48,-3.49,;-.57,-2.24,;-1.48,-1,;-2.94,-1.47,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600003
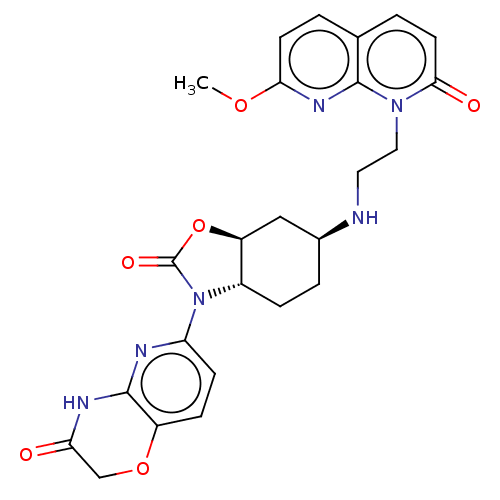
(CHEMBL5201228)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ccc1=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50463771

(CHEMBL4250651)Show SMILES CNc1cc(F)cc2c1[nH]c1nc(CNC(=O)c3cnco3)nc(N3CCC(N)C3)c21 Show InChI InChI=1S/C20H21FN8O2/c1-23-13-5-10(21)4-12-16-18(28-17(12)13)26-15(7-25-20(30)14-6-24-9-31-14)27-19(16)29-3-2-11(22)8-29/h4-6,9,11,23H,2-3,7-8,22H2,1H3,(H,25,30)(H,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Redx Pharma
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DNA gyrase ATPase activity |
Bioorg Med Chem Lett 28: 2998-3003 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.049
BindingDB Entry DOI: 10.7270/Q2G73HD3 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50050549

(GSK2140944 | Gepotidacin)Show SMILES O=c1ccc2ncc(=O)n3[C@H](CN4CCC(CC4)NCc4cc5CCCOc5cn4)Cn1c23 |r| Show InChI InChI=1S/C24H28N6O3/c31-22-4-3-20-24-29(22)15-19(30(24)23(32)13-27-20)14-28-7-5-17(6-8-28)25-11-18-10-16-2-1-9-33-21(16)12-26-18/h3-4,10,12-13,17,19,25H,1-2,5-9,11,14-15H2/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50226181

((3R,4S,5R,6R)-5-hydroxy-6-(4-hydroxy-3-(4-hydroxy-...)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@@H](-[#8]-[#6](-[#7])=O)-[#6@@H](-[#8])-[#6@H](-[#8]-c2ccc3c(-[#8])c(-[#7]-[#6](=O)-c4ccc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6])c4)c(=O)oc3c2-[#6])-[#8]C1([#6])[#6] |r| Show InChI InChI=1S/C31H36N2O11/c1-14(2)7-8-16-13-17(9-11-19(16)34)27(37)33-21-22(35)18-10-12-20(15(3)24(18)42-28(21)38)41-29-23(36)25(43-30(32)39)26(40-6)31(4,5)44-29/h7,9-13,23,25-26,29,34-36H,8H2,1-6H3,(H2,32,39)(H,33,37)/t23-,25+,26-,29-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Redx Pharma
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DNA gyrase activity |
Bioorg Med Chem Lett 28: 2998-3003 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.049
BindingDB Entry DOI: 10.7270/Q2G73HD3 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600006

(CHEMBL5172158)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2cc(cnc2ccc1=O)C#N |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600007
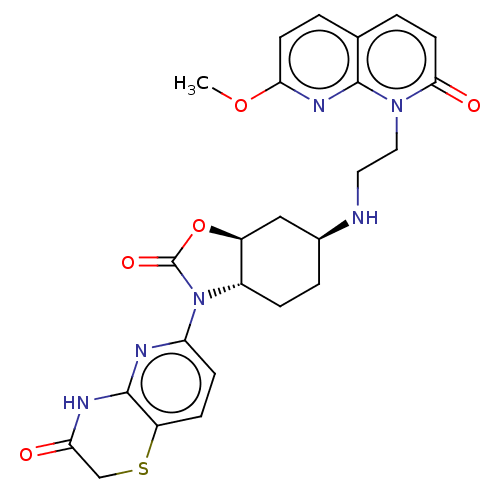
(CHEMBL5182200)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2SCC(=O)Nc2n1)NCCn1c2nc(OC)ccc2ccc1=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 194 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50553249
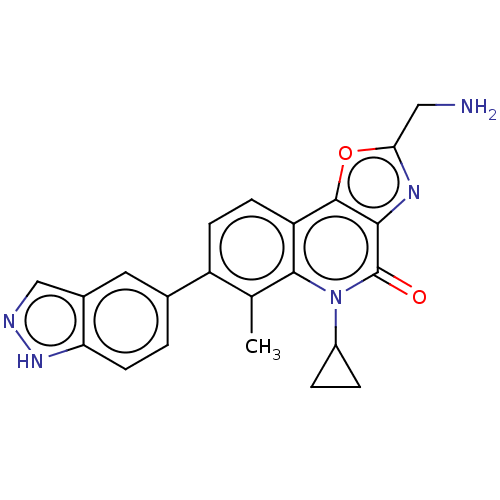
(CHEMBL4743440)Show SMILES Cc1c(ccc2c3oc(CN)nc3c(=O)n(C3CC3)c12)-c1ccc2[nH]ncc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1039/d0md00174k
BindingDB Entry DOI: 10.7270/Q2WH2TN2 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600004
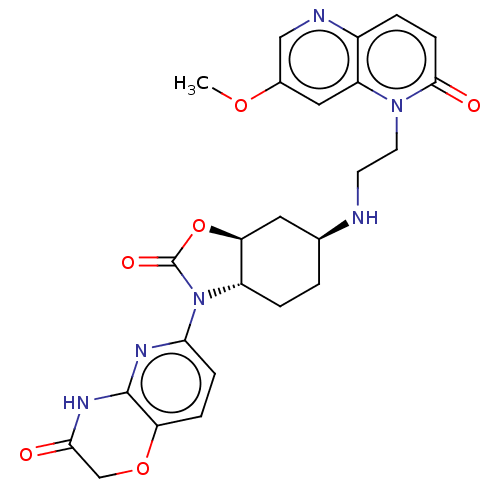
(CHEMBL5184104)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCn1c2cc(OC)cnc2ccc1=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM159756

(PF-02384554 | US10966980, Example 8 | US9035074, 8)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1cc(ccn1)C#N)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-5.61,1.61,;-4.28,.84,;-2.94,1.61,;-2.55,3.09,;-1.06,2.69,;-1.46,1.21,;.28,3.47,;1.61,2.69,;.52,1.61,;2.7,3.78,;2.94,1.93,;2.94,.38,;4.28,-.38,;5.61,.38,;5.61,1.93,;4.28,2.69,;4.28,-1.92,;4.28,-3.46,;-4.28,-.7,;-5.61,-1.47,;-5.61,-3.01,;-4.28,-3.78,;-2.94,-3.01,;-1.48,-3.49,;-.57,-2.24,;-1.48,-1,;-2.94,-1.47,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50553247
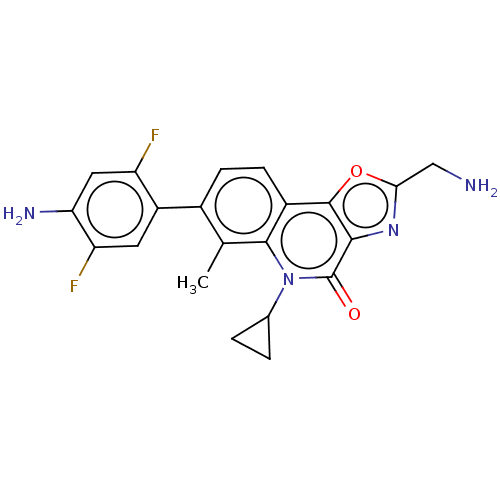
(CHEMBL4764598)Show SMILES Cc1c(ccc2c3oc(CN)nc3c(=O)n(C3CC3)c12)-c1cc(F)c(N)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1039/d0md00174k
BindingDB Entry DOI: 10.7270/Q2WH2TN2 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600009
(CHEMBL5177076)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCCn1c2cc(cnc2ccc1=O)C#N |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50600008
(CHEMBL5198597)Show SMILES [H][C@]12C[C@H](CC[C@]1([H])N(C(=O)O2)c1ccc2OCC(=O)Nc2n1)NCCCn1c2cc(OC)cnc2ccc1=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128648
BindingDB Entry DOI: 10.7270/Q2MC942Z |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 472 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50553247

(CHEMBL4764598)Show SMILES Cc1c(ccc2c3oc(CN)nc3c(=O)n(C3CC3)c12)-c1cc(F)c(N)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1039/d0md00174k
BindingDB Entry DOI: 10.7270/Q2WH2TN2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 592 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM159756

(PF-02384554 | US10966980, Example 8 | US9035074, 8)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1cc(ccn1)C#N)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-5.61,1.61,;-4.28,.84,;-2.94,1.61,;-2.55,3.09,;-1.06,2.69,;-1.46,1.21,;.28,3.47,;1.61,2.69,;.52,1.61,;2.7,3.78,;2.94,1.93,;2.94,.38,;4.28,-.38,;5.61,.38,;5.61,1.93,;4.28,2.69,;4.28,-1.92,;4.28,-3.46,;-4.28,-.7,;-5.61,-1.47,;-5.61,-3.01,;-4.28,-3.78,;-2.94,-3.01,;-1.48,-3.49,;-.57,-2.24,;-1.48,-1,;-2.94,-1.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 605 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 606 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 608 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM159756

(PF-02384554 | US10966980, Example 8 | US9035074, 8)Show SMILES CN([C@@H]1C[C@@H](C1)NS(=O)(=O)c1cc(ccn1)C#N)c1ncnc2[nH]ccc12 |r,wD:2.1,4.6,(-5.61,1.61,;-4.28,.84,;-2.94,1.61,;-2.55,3.09,;-1.06,2.69,;-1.46,1.21,;.28,3.47,;1.61,2.69,;.52,1.61,;2.7,3.78,;2.94,1.93,;2.94,.38,;4.28,-.38,;5.61,.38,;5.61,1.93,;4.28,2.69,;4.28,-1.92,;4.28,-3.46,;-4.28,-.7,;-5.61,-1.47,;-5.61,-3.01,;-4.28,-3.78,;-2.94,-3.01,;-1.48,-3.49,;-.57,-2.24,;-1.48,-1,;-2.94,-1.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50553249

(CHEMBL4743440)Show SMILES Cc1c(ccc2c3oc(CN)nc3c(=O)n(C3CC3)c12)-c1ccc2[nH]ncc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1039/d0md00174k
BindingDB Entry DOI: 10.7270/Q2WH2TN2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50553248

(CHEMBL4787847)Show SMILES Cc1c(ccc2c3oc(CN)nc3c(=O)n(C3CC3)c12)-c1cnc2[nH]ncc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1039/d0md00174k
BindingDB Entry DOI: 10.7270/Q2WH2TN2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50553249

(CHEMBL4743440)Show SMILES Cc1c(ccc2c3oc(CN)nc3c(=O)n(C3CC3)c12)-c1ccc2[nH]ncc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1039/d0md00174k
BindingDB Entry DOI: 10.7270/Q2WH2TN2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D
| Assay Description
Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data