

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50096629 (3-(4-Amino-cyclohexyl)-2-oxo-3-[((6S,8aS)-4-oxo-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072528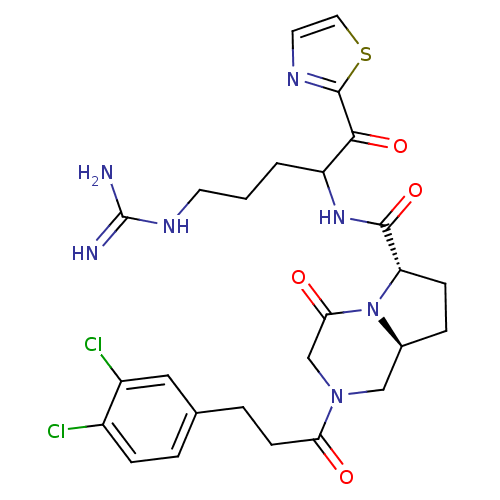 ((6S,8aS)-2-[3-(3,4-Dichloro-phenyl)-propionyl]-4-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096623 (3-(4-Amino-cyclohexyl)-2-oxo-3-[(4-oxo-2-phenylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096628 (3-(4-Amino-cyclohexyl)-2-oxo-3-[((6S,8aS)-4-oxo-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50160248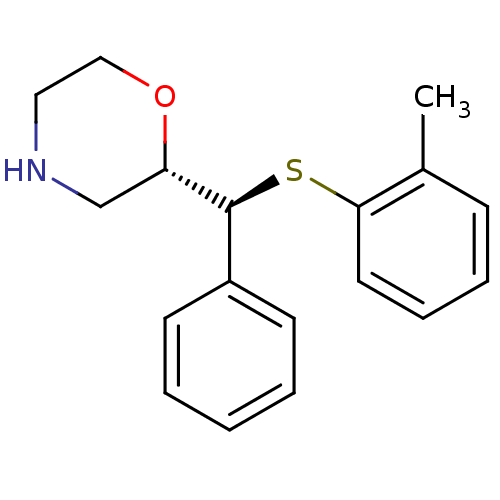 ((2S,3S)-2-[alpha-(2-Methylphenylthio)phenylmethyl]...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET receptor expressed in HEK293 cells | J Med Chem 52: 62-73 (2009) Article DOI: 10.1021/jm800817h BindingDB Entry DOI: 10.7270/Q2CN74TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096620 ((6S,8aS)-4-Oxo-2-phenylmethanesulfonyl-octahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072536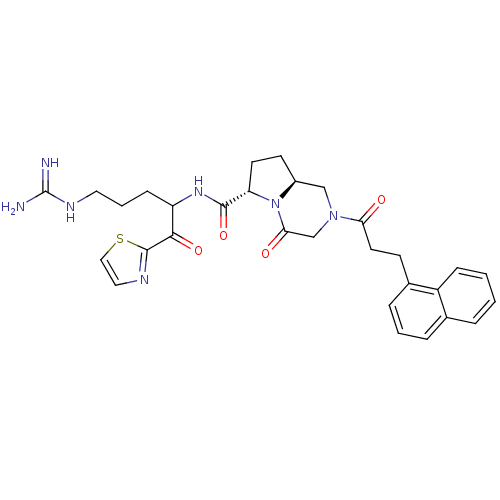 ((6S,8aS)-2-(3-Naphthalen-1-yl-propionyl)-4-oxo-oct...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14774 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072524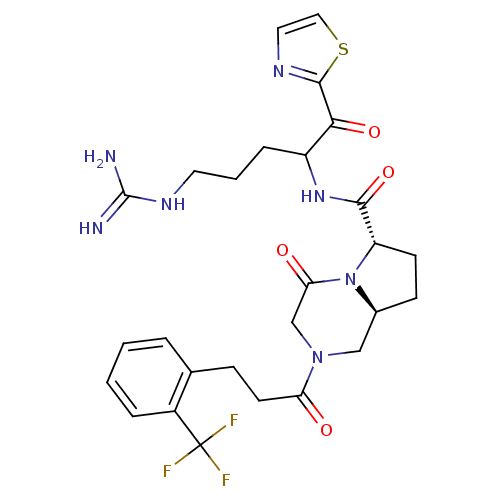 ((6S,8aS)-4-Oxo-2-[3-(2-trifluoromethyl-phenyl)-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072527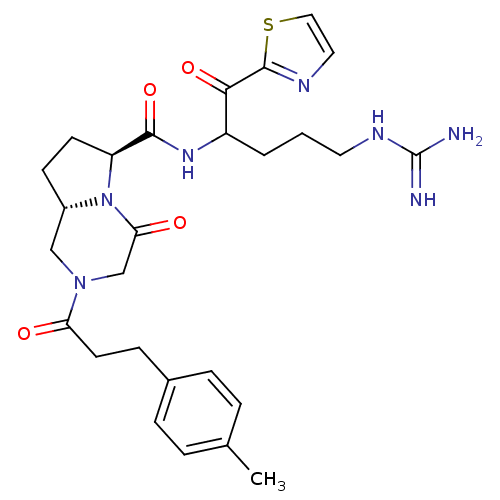 ((6S,8aS)-4-Oxo-2-(3-p-tolyl-propionyl)-octahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072537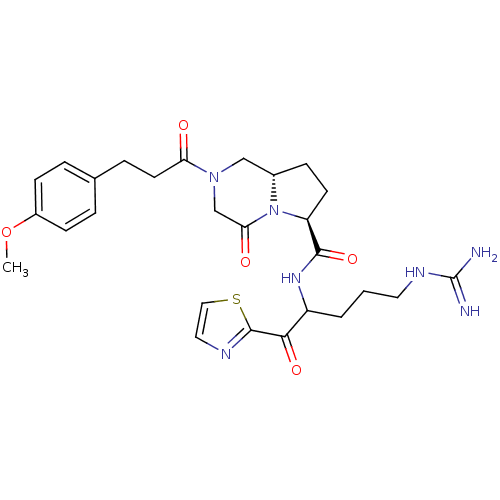 ((6S,8aS)-2-[3-(4-Methoxy-phenyl)-propionyl]-4-oxo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072534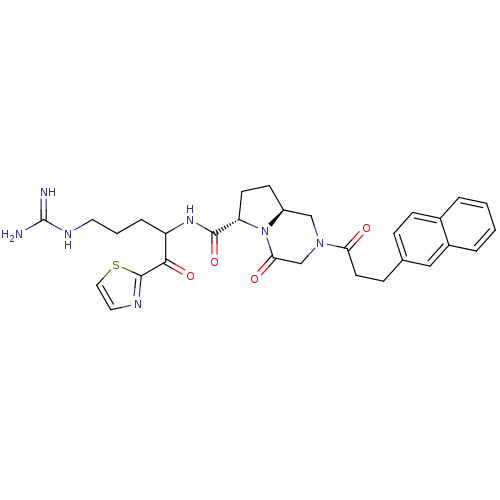 ((6S,8aS)-2-(3-Naphthalen-2-yl-propionyl)-4-oxo-oct...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Rattus norvegicus) | BDBM50072740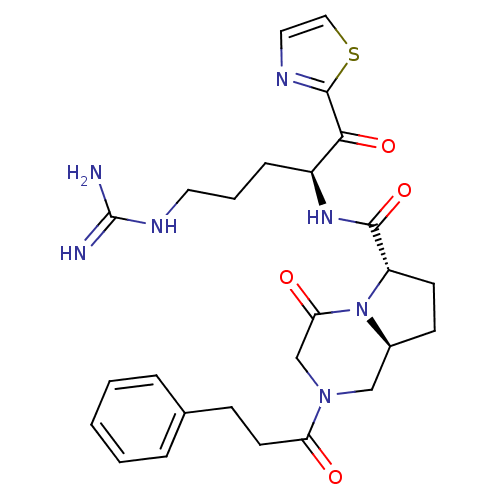 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the amidolytic activity of thrombin | Bioorg Med Chem Lett 11: 3161-4 (2001) BindingDB Entry DOI: 10.7270/Q2JM28XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072740 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound towards Thrombin | J Med Chem 43: 361-8 (2000) BindingDB Entry DOI: 10.7270/Q2P26XCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072532 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50427452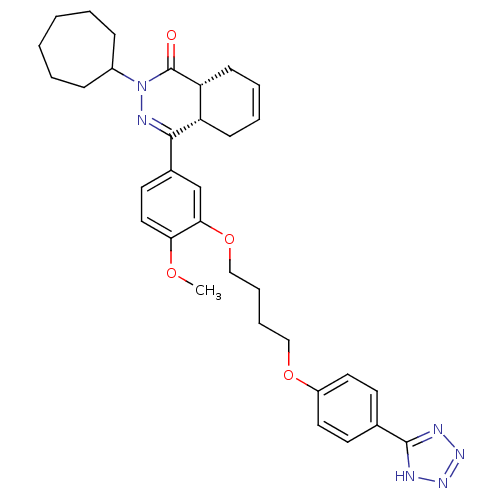 (CHEMBL2326941) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347563 (CHEMBL1801740) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human Angiotensin receptor 1 | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096621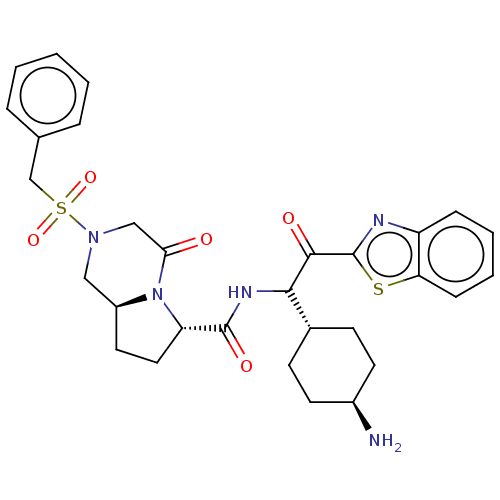 ((6S,8aS)-4-Oxo-2-phenylmethanesulfonyl-octahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072520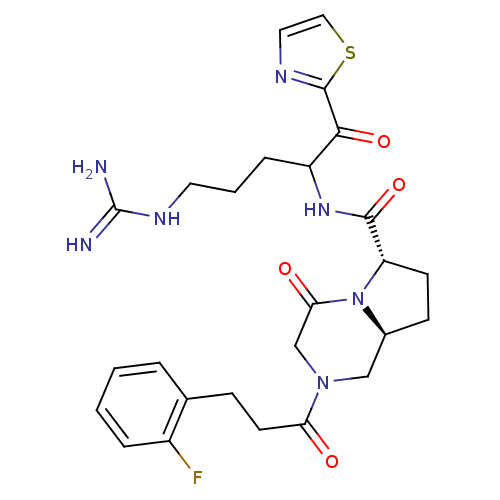 ((6S,8aS)-2-[3-(2-Fluoro-phenyl)-propionyl]-4-oxo-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50199005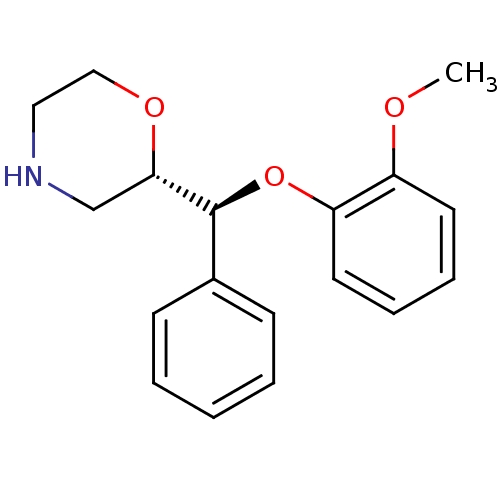 ((S)-2-((S)-(2-methoxyphenoxy)(phenyl)methyl)morpho...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET receptor expressed in HEK293 cells | J Med Chem 52: 62-73 (2009) Article DOI: 10.1021/jm800817h BindingDB Entry DOI: 10.7270/Q2CN74TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111673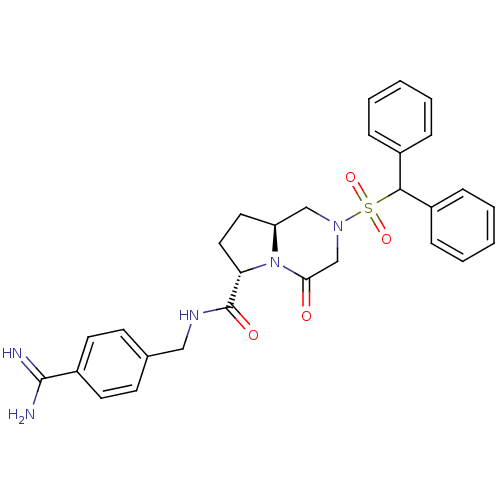 ((6S,8aS)-2-(Diphenyl-methanesulfonyl)-4-oxo-octahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111661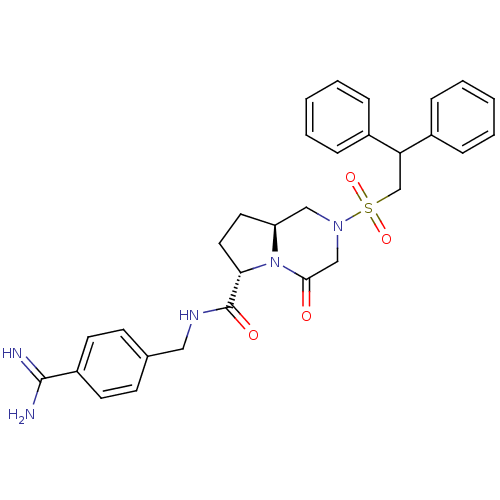 ((6S,8aS)-2-(2,2-Diphenyl-ethanesulfonyl)-4-oxo-oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50254209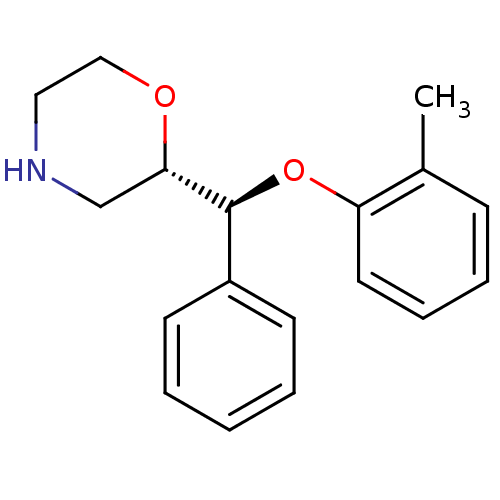 ((2S,3S)-2-[alpha-(2-Methylphenoxy)phenylmethyl]mor...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET receptor expressed in HEK293 cells | J Med Chem 52: 62-73 (2009) Article DOI: 10.1021/jm800817h BindingDB Entry DOI: 10.7270/Q2CN74TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50370475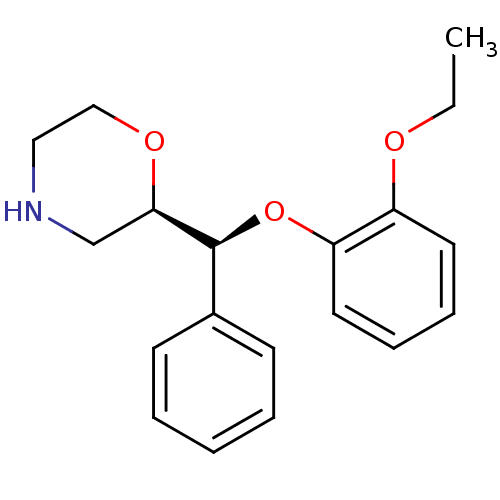 (S,S-REBOXETINE) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET receptor expressed in HEK293 cells | J Med Chem 52: 62-73 (2009) Article DOI: 10.1021/jm800817h BindingDB Entry DOI: 10.7270/Q2CN74TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401002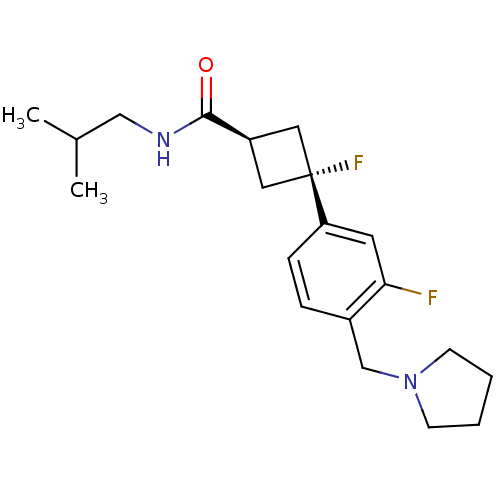 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080924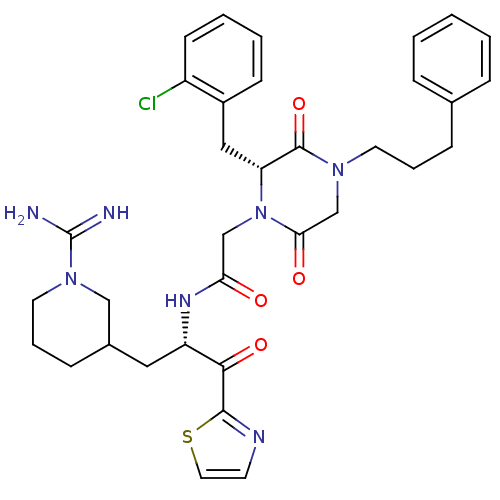 (CHEMBL83260 | N-[(S)-1-(1-Carbamimidoyl-piperidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 2503-8 (1999) BindingDB Entry DOI: 10.7270/Q2028QRW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072533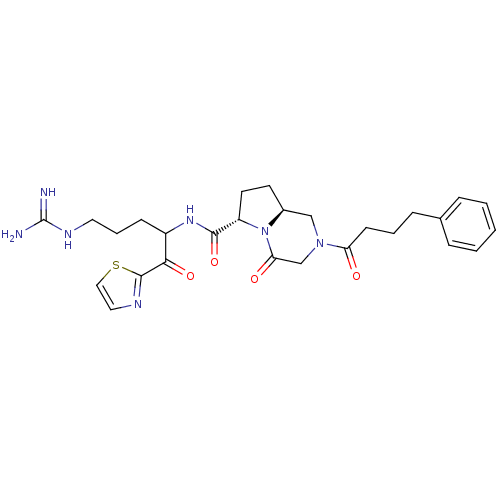 ((6S,8aS)-4-Oxo-2-(4-phenyl-butyryl)-octahydro-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401007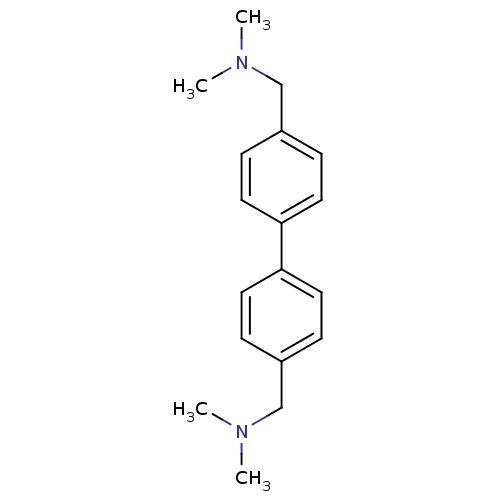 (CHEMBL209478) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401007 (CHEMBL209478) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401004 (CHEMBL2206291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401004 (CHEMBL2206291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401003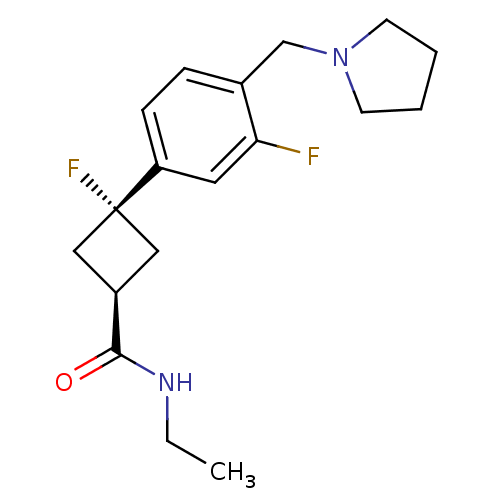 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401003 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111675 ((6S,8aS)-2-(2,2-Diphenyl-ethanesulfonyl)-4-oxo-oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111658 ((6S,8aS)-2-(Diphenyl-methanesulfonyl)-4-oxo-octahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111665 ((6S,8aS)-2-(Diphenyl-methanesulfonyl)-4-oxo-octahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111672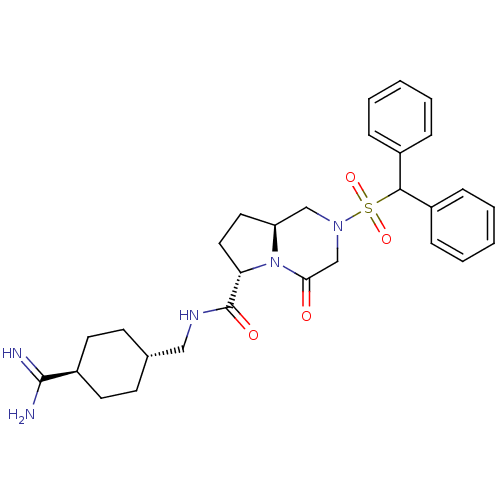 ((6S,8aS)-2-(Diphenyl-methanesulfonyl)-4-oxo-octahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem. Curated by ChEMBL | Assay Description Inhibition of amidolytic activity of thrombin | Bioorg Med Chem Lett 12: 1181-4 (2002) BindingDB Entry DOI: 10.7270/Q22V2FFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096622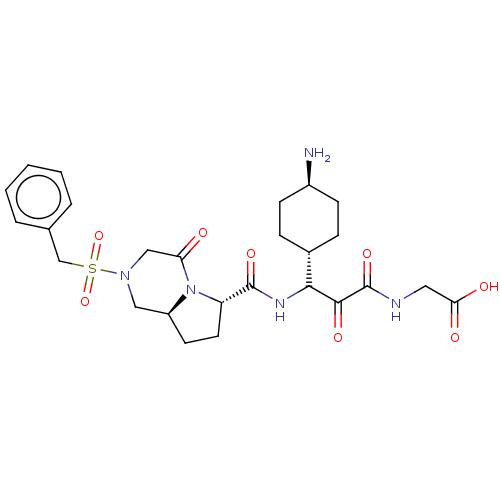 (CHEMBL3084834 | {3-(4-Amino-cyclohexyl)-2-oxo-3-[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401003 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401003 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072530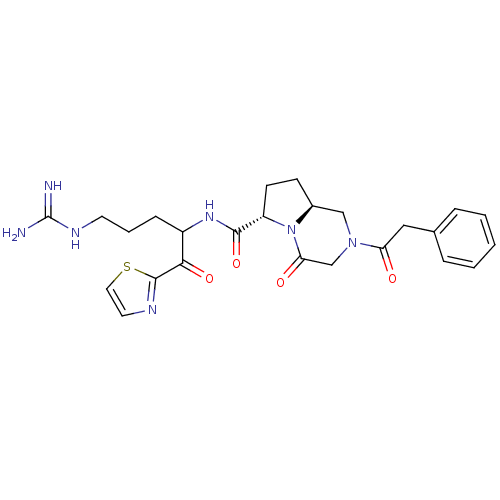 ((6S,8aS)-4-Oxo-2-phenylacetyl-octahydro-pyrrolo[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072541 ((6S,8aS)-4-Oxo-2-(3-pyridin-2-yl-propionyl)-octahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072519 ((6S,8aS)-2-[(S)-2-Amino-3-(4-fluoro-phenyl)-propio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound against thrombin | Bioorg Med Chem Lett 8: 3193-8 (1999) BindingDB Entry DOI: 10.7270/Q2HT2NGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8326 total ) | Next | Last >> |