

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050693 (CHEMBL3311539) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Mixed type inhibition of Torpedo californica AChE by Lineweaver-Burk plot | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019010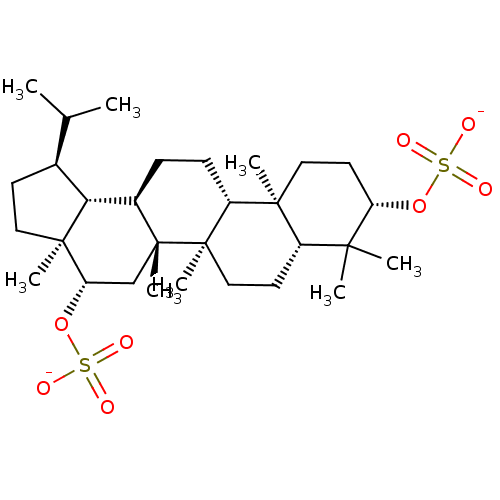 (CHEMBL3288082) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Uncompetitive inhibition of Pacific electric ray AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050693 (CHEMBL3311539) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050692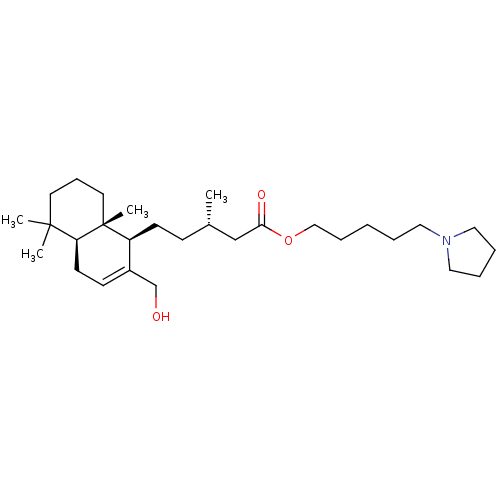 (CHEMBL3311540) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050569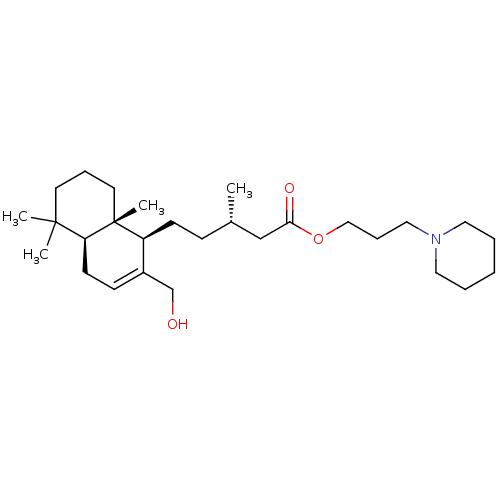 (CHEMBL3311548) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050573 (CHEMBL3311544) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050693 (CHEMBL3311539) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050566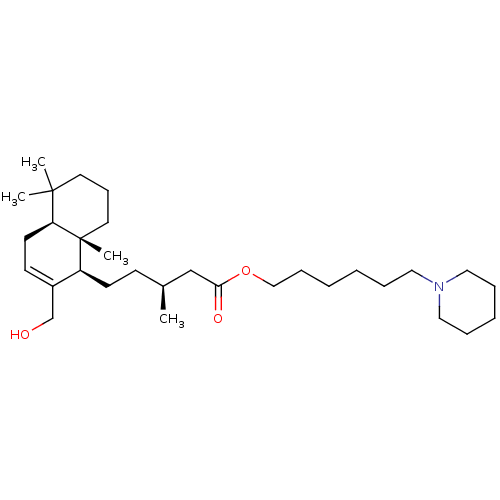 (CHEMBL3309346) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050691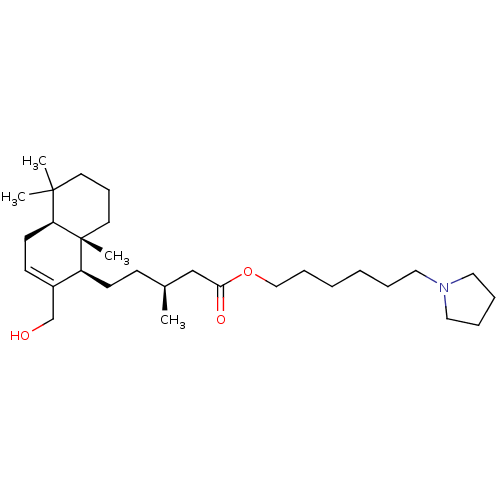 (CHEMBL3311541) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050689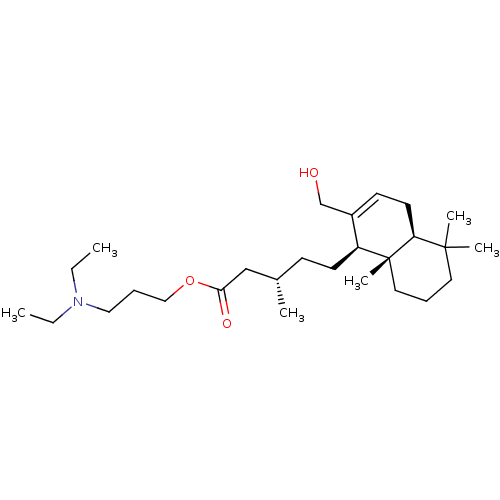 (CHEMBL3311543) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050691 (CHEMBL3311541) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050572 (CHEMBL3311545) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050566 (CHEMBL3309346) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050567 (CHEMBL3311550) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050568 (CHEMBL3311549) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050571 (CHEMBL3311546) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050695 (CHEMBL3311457) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050571 (CHEMBL3311546) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050689 (CHEMBL3311543) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050696 (CHEMBL3311456) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019013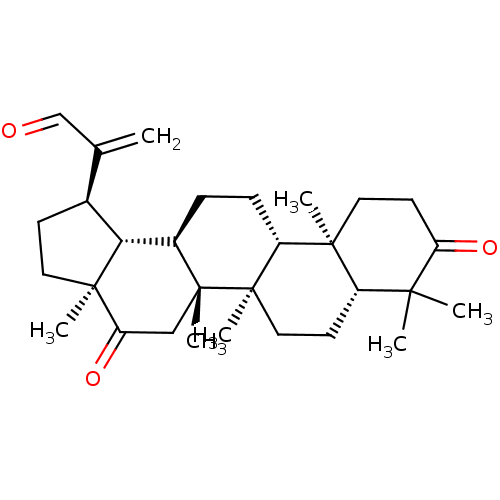 (CHEMBL3288094) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050692 (CHEMBL3311540) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050573 (CHEMBL3311544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050567 (CHEMBL3311550) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050572 (CHEMBL3311545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050694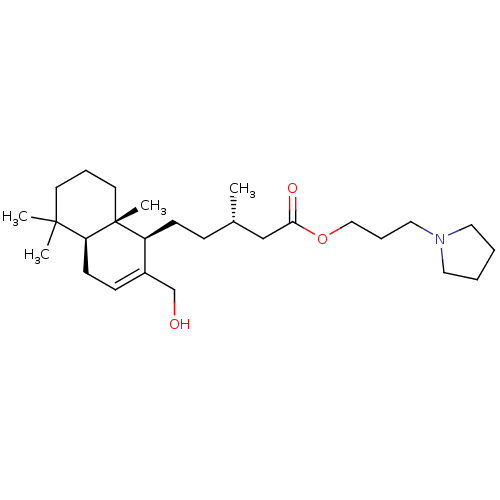 (CHEMBL3311458) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050569 (CHEMBL3311548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050568 (CHEMBL3311549) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050690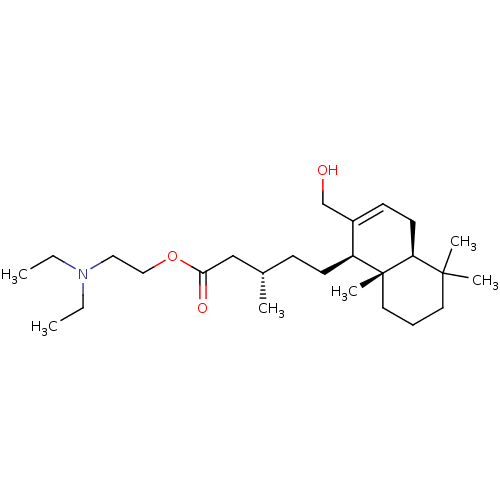 (CHEMBL3311542) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050694 (CHEMBL3311458) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050565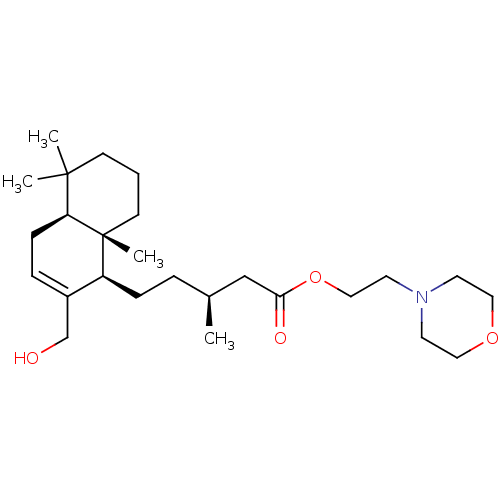 (CHEMBL3309347) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050564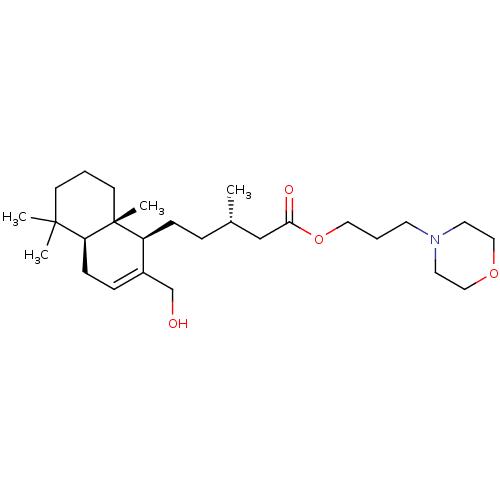 (CHEMBL3309348) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050563 (CHEMBL3309349) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050562 (CHEMBL3309350) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050561 (CHEMBL3309351) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050570 (CHEMBL3311547) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050690 (CHEMBL3311542) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50050695 (CHEMBL3311457) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050561 (CHEMBL3309351) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050562 (CHEMBL3309350) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050563 (CHEMBL3309349) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050564 (CHEMBL3309348) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050565 (CHEMBL3309347) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50050570 (CHEMBL3311547) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of horse serum BChE by spectrophotometry based Ellman's method | Bioorg Med Chem 22: 3838-49 (2014) Article DOI: 10.1016/j.bmc.2014.06.030 BindingDB Entry DOI: 10.7270/Q2VT1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 73 total ) | Next | Last >> |