 Found 71 hits with Last Name = 'nagar' and Initial = 'm'
Found 71 hits with Last Name = 'nagar' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM50276880
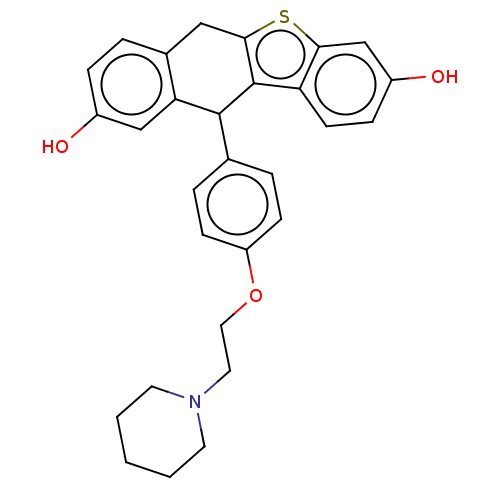
(CHEMBL4171598)Show SMILES Oc1ccc2Cc3sc4cc(O)ccc4c3C(c3ccc(OCCN4CCCCC4)cc3)c2c1 Show InChI InChI=1S/C29H29NO3S/c31-21-7-4-20-16-27-29(24-11-8-22(32)18-26(24)34-27)28(25(20)17-21)19-5-9-23(10-6-19)33-15-14-30-12-2-1-3-13-30/h4-11,17-18,28,31-32H,1-3,12-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha (unknown origin) |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50276880

(CHEMBL4171598)Show SMILES Oc1ccc2Cc3sc4cc(O)ccc4c3C(c3ccc(OCCN4CCCCC4)cc3)c2c1 Show InChI InChI=1S/C29H29NO3S/c31-21-7-4-20-16-27-29(24-11-8-22(32)18-26(24)34-27)28(25(20)17-21)19-5-9-23(10-6-19)33-15-14-30-12-2-1-3-13-30/h4-11,17-18,28,31-32H,1-3,12-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Binding affinity to ERbeta (unknown origin) |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50276892
(CHEMBL4167943)Show InChI InChI=1S/C14H10OS/c15-12-7-5-10(6-8-12)14-9-11-3-1-2-4-13(11)16-14/h1-9,15H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of [125I]2-(3'-Iodo-4'-N-methylaminophenyl) benzothiazole binding to amyloid beta (1 to 40) (unknown origin) after 3 hrs by NaI well count... |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50276892

(CHEMBL4167943)Show InChI InChI=1S/C14H10OS/c15-12-7-5-10(6-8-12)14-9-11-3-1-2-4-13(11)16-14/h1-9,15H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of [125I]2-(3'-Iodo-4'-N-methylaminophenyl) benzothiazole binding to amyloid beta (1 to 42) (unknown origin) after 3 hrs by NaI well count... |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50276883
(CHEMBL4175800)Show InChI InChI=1S/C14H11NS/c15-12-7-5-10(6-8-12)14-9-11-3-1-2-4-13(11)16-14/h1-9H,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of [125I]2-(3'-Iodo-4'-N-methylaminophenyl) benzothiazole binding to amyloid beta (1 to 40) (unknown origin) after 3 hrs by NaI well count... |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50276883
(CHEMBL4175800)Show InChI InChI=1S/C14H11NS/c15-12-7-5-10(6-8-12)14-9-11-3-1-2-4-13(11)16-14/h1-9H,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of [125I]2-(3'-Iodo-4'-N-methylaminophenyl) benzothiazole binding to amyloid beta (1 to 40) (unknown origin) after 3 hrs by NaI well count... |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50276901
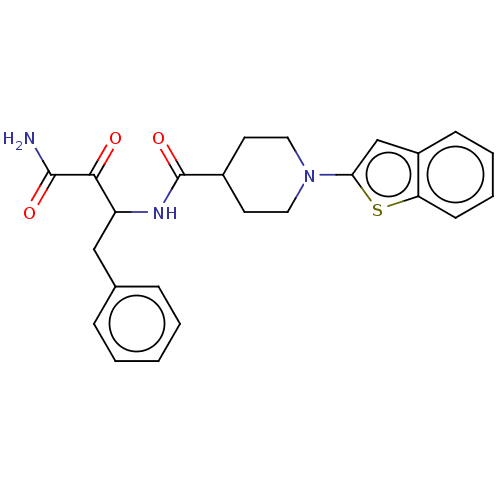
(CHEMBL4167219)Show SMILES NC(=O)C(=O)C(Cc1ccccc1)NC(=O)C1CCN(CC1)c1cc2ccccc2s1 Show InChI InChI=1S/C24H25N3O3S/c25-23(29)22(28)19(14-16-6-2-1-3-7-16)26-24(30)17-10-12-27(13-11-17)21-15-18-8-4-5-9-20(18)31-21/h1-9,15,17,19H,10-14H2,(H2,25,29)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes mu-calpain using SucLeu-Tyr-AMC as substrate |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Proline racemase
(Clostridium sticklandii) | BDBM50444845

(CHEMBL1235532)Show InChI InChI=1S/C5H5NO2/c7-5(8)4-2-1-3-6-4/h1-3,6H,(H,7,8)/p-1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal 6xHis-tagged Clostridium sticklandii ATCC 12662 proline racemase expressed in Escherichia coli BL21(DE3) using L... |
Bioorg Med Chem Lett 24: 390-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.061
BindingDB Entry DOI: 10.7270/Q2PV6MVC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50276899
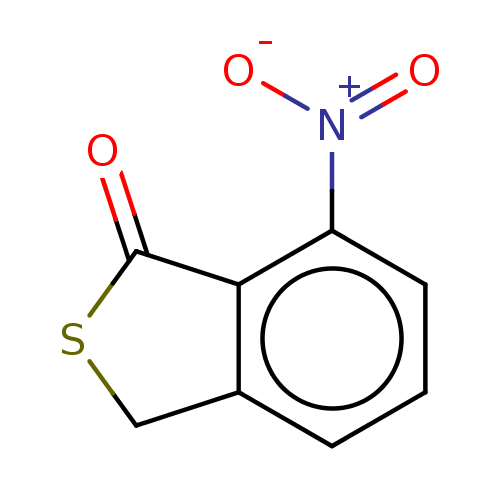
(CHEMBL4166725)Show InChI InChI=1S/C8H5NO3S/c10-8-7-5(4-13-8)2-1-3-6(7)9(11)12/h1-3H,4H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50276882
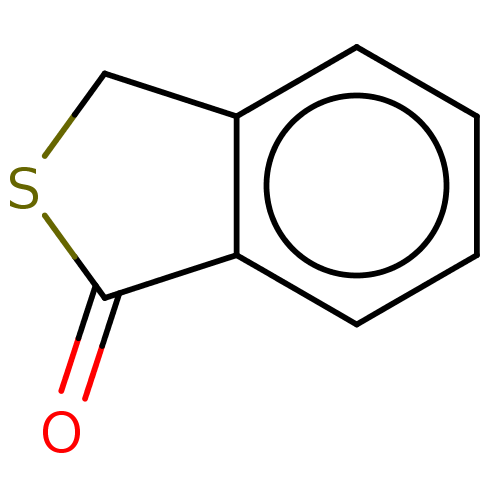
(CHEMBL4162224)Show InChI InChI=1S/C8H6OS/c9-8-7-4-2-1-3-6(7)5-10-8/h1-4H,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50276899

(CHEMBL4166725)Show InChI InChI=1S/C8H5NO3S/c10-8-7-5(4-13-8)2-1-3-6(7)9(11)12/h1-3H,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Mandelate racemase
(Pseudomonas putida (g-Proteobacteria)) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Pseudomonas putida mandelate racemase |
Bioorg Med Chem Lett 24: 390-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.061
BindingDB Entry DOI: 10.7270/Q2PV6MVC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mandelate racemase
(Pseudomonas putida (g-Proteobacteria)) | BDBM50273974
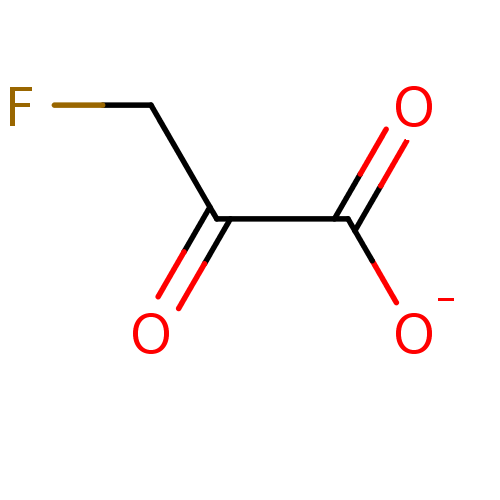
(3-fluoro-2-oxopropanoate)Show InChI InChI=1S/C3H3FO3/c4-1-2(5)3(6)7/h1H2,(H,6,7)/p-1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Dalhousie University
| Assay Description
MR activity was assayed using a CD-based assay by following the change in ellipticity of mandelate at 262 nm using a thermostated quartz cuvette with... |
Biochemistry 54: 2747-57 (2015)
Article DOI: 10.1021/acs.biochem.5b00221
BindingDB Entry DOI: 10.7270/Q2ZK5FDT |
More data for this
Ligand-Target Pair | |
Mandelate racemase
(Pseudomonas putida (g-Proteobacteria)) | BDBM152705
(Mesoxalate)Show InChI InChI=1S/C3H2O5/c4-1(2(5)6)3(7)8/h(H,5,6)(H,7,8)/p-2 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Dalhousie University
| Assay Description
MR activity was assayed using a CD-based assay by following the change in ellipticity of mandelate at 262 nm using a thermostated quartz cuvette with... |
Biochemistry 54: 2747-57 (2015)
Article DOI: 10.1021/acs.biochem.5b00221
BindingDB Entry DOI: 10.7270/Q2ZK5FDT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50276882

(CHEMBL4162224)Show InChI InChI=1S/C8H6OS/c9-8-7-4-2-1-3-6(7)5-10-8/h1-4H,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.89E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of BuChE (unknown origin) |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Proline racemase
(Clostridium sticklandii) | BDBM50444846
(CHEMBL3099361)Show InChI InChI=1S/C8H13NO2/c10-7(11)8-3-1-5-9(8)6-2-4-8/h1-6H2,(H,10,11) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.11E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of N-terminal 6xHis-tagged Clostridium sticklandii ATCC 12662 proline racemase expressed in Escherichia coli BL21(DE3) usin... |
Bioorg Med Chem Lett 24: 390-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.061
BindingDB Entry DOI: 10.7270/Q2PV6MVC |
More data for this
Ligand-Target Pair | |
Serine racemase
(Schizosaccharomyces pombe (strain 972 / ATCC 24843...) | BDBM50444848

(CHEMBL3099359)Show InChI InChI=1S/C4H9NO4/c5-4(1-6,2-7)3(8)9/h6-7H,1-2,5H2,(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.67E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of N-terminal 6xHis-tagged recombinant Schizosaccharomyces pombe 972 serine racemase expressed in Escherichia coli BL21(DE3) us... |
Bioorg Med Chem Lett 24: 390-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.061
BindingDB Entry DOI: 10.7270/Q2PV6MVC |
More data for this
Ligand-Target Pair | |
Serine racemase
(Schizosaccharomyces pombe (strain 972 / ATCC 24843...) | BDBM50444848

(CHEMBL3099359)Show InChI InChI=1S/C4H9NO4/c5-4(1-6,2-7)3(8)9/h6-7H,1-2,5H2,(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.61E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of N-terminal 6xHis-tagged recombinant Schizosaccharomyces pombe 972 serine racemase expressed in Escherichia coli BL21(DE3) us... |
Bioorg Med Chem Lett 24: 390-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.061
BindingDB Entry DOI: 10.7270/Q2PV6MVC |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50276884
(CHEMBL42707)Show SMILES Oc1ccc(cc1)-c1sc2cccc(O)c2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-11-7-20(8-12-21)28-26(25-23(31)5-4-6-24(25)34-28)27(32)19-9-13-22(14-10-19)33-18-17-29-15-2-1-3-16-29/h4-14,30-31H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Displacement of [3H]17beta-estradiol from ER in human MCF7 cells after 18 hrs by microbeta scintillation counting method |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50276893
(CHEMBL1193539)Show SMILES Oc1ccc2c(C(=O)c3ccc(OCCN4CCCCC4)cc3)c(sc2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H26ClNO3S/c29-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-30-14-2-1-3-15-30/h4-13,18,31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Displacement of [3H]17beta-estradiol from ER in human MCF7 cells after 18 hrs by microbeta scintillation counting method |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50276880

(CHEMBL4171598)Show SMILES Oc1ccc2Cc3sc4cc(O)ccc4c3C(c3ccc(OCCN4CCCCC4)cc3)c2c1 Show InChI InChI=1S/C29H29NO3S/c31-21-7-4-20-16-27-29(24-11-8-22(32)18-26(24)34-27)28(25(20)17-21)19-5-9-23(10-6-19)33-15-14-30-12-2-1-3-13-30/h4-11,17-18,28,31-32H,1-3,12-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Antagonist activity at ER in human MCF7 cells assessed as inhibition of 17beta-estradiol-induced cell proliferation |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM16452

((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(ccc3s2)C(F)(F)F)c(=O)c2ccccc12 Show InChI InChI=1S/C19H12F3N3O3S/c20-19(21,22)10-5-6-15-14(7-10)23-16(29-15)9-25-18(28)12-4-2-1-3-11(12)13(24-25)8-17(26)27/h1-7H,8-9H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of human placental aldose reductase using glyceraldehyde as substrate in presence of NADPH |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50276900
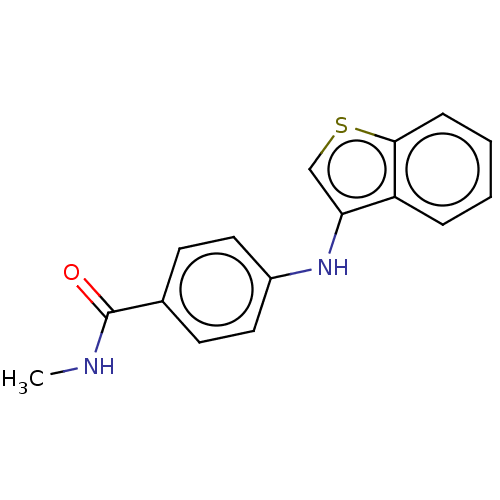
(CHEMBL4168213)Show InChI InChI=1S/C16H14N2OS/c1-17-16(19)11-6-8-12(9-7-11)18-14-10-20-15-5-3-2-4-13(14)15/h2-10,18H,1H3,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50362408
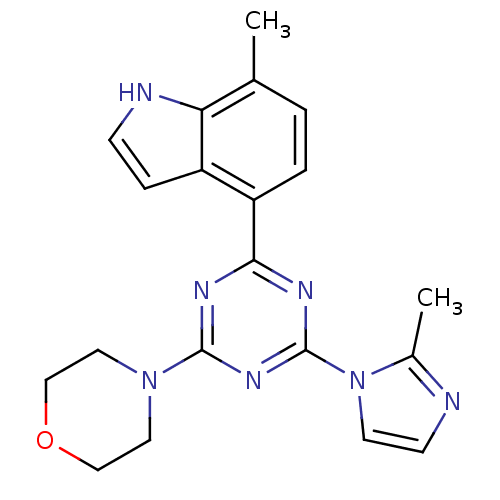
(CHEMBL1940142)Show SMILES Cc1nccn1-c1nc(nc(n1)-c1ccc(C)c2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C20H21N7O/c1-13-3-4-16(15-5-6-22-17(13)15)18-23-19(26-9-11-28-12-10-26)25-20(24-18)27-8-7-21-14(27)2/h3-8,22H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50362405
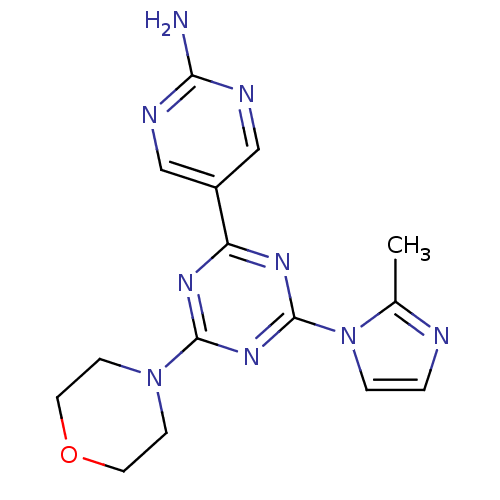
(CHEMBL1940139)Show InChI InChI=1S/C15H17N9O/c1-10-17-2-3-24(10)15-21-12(11-8-18-13(16)19-9-11)20-14(22-15)23-4-6-25-7-5-23/h2-3,8-9H,4-7H2,1H3,(H2,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315213
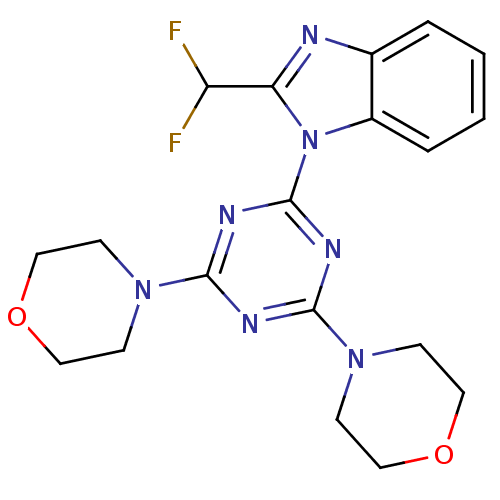
(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50362406

(CHEMBL1940140)Show SMILES CCc1nccn1-c1nc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C20H21N7O/c1-2-17-22-8-9-27(17)20-24-18(15-4-3-5-16-14(15)6-7-21-16)23-19(25-20)26-10-12-28-13-11-26/h3-9,21H,2,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50276880

(CHEMBL4171598)Show SMILES Oc1ccc2Cc3sc4cc(O)ccc4c3C(c3ccc(OCCN4CCCCC4)cc3)c2c1 Show InChI InChI=1S/C29H29NO3S/c31-21-7-4-20-16-27-29(24-11-8-22(32)18-26(24)34-27)28(25(20)17-21)19-5-9-23(10-6-19)33-15-14-30-12-2-1-3-13-30/h4-11,17-18,28,31-32H,1-3,12-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Antagonist activity at ER in human Ishikawa cells assessed as inhibition of E2-induced alkaline phosphatase induction after 72 hrs by PNPP substrate ... |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50362404
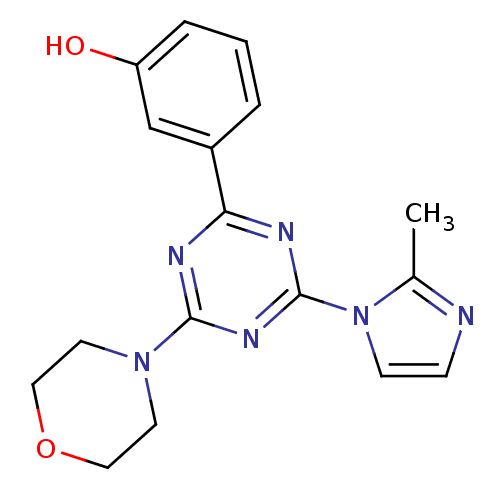
(CHEMBL1940138)Show InChI InChI=1S/C17H18N6O2/c1-12-18-5-6-23(12)17-20-15(13-3-2-4-14(24)11-13)19-16(21-17)22-7-9-25-10-8-22/h2-6,11,24H,7-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50362407
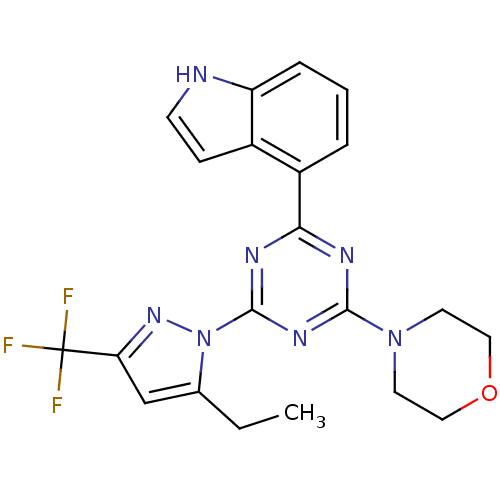
(CHEMBL1940141)Show SMILES CCc1cc(nn1-c1nc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C21H20F3N7O/c1-2-13-12-17(21(22,23)24)29-31(13)20-27-18(15-4-3-5-16-14(15)6-7-25-16)26-19(28-20)30-8-10-32-11-9-30/h3-7,12,25H,2,8-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50362408

(CHEMBL1940142)Show SMILES Cc1nccn1-c1nc(nc(n1)-c1ccc(C)c2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C20H21N7O/c1-13-3-4-16(15-5-6-22-17(13)15)18-23-19(26-9-11-28-12-10-26)25-20(24-18)27-8-7-21-14(27)2/h3-8,22H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mTOR-mediated p70S6K phosphorylation at Thr389 in human PC3 cells |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50362406

(CHEMBL1940140)Show SMILES CCc1nccn1-c1nc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C20H21N7O/c1-2-17-22-8-9-27(17)20-24-18(15-4-3-5-16-14(15)6-7-21-16)23-19(25-20)26-10-12-28-13-11-26/h3-9,21H,2,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50362404

(CHEMBL1940138)Show InChI InChI=1S/C17H18N6O2/c1-12-18-5-6-23(12)17-20-15(13-3-2-4-14(24)11-13)19-16(21-17)22-7-9-25-10-8-22/h2-6,11,24H,7-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50362406

(CHEMBL1940140)Show SMILES CCc1nccn1-c1nc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C20H21N7O/c1-2-17-22-8-9-27(17)20-24-18(15-4-3-5-16-14(15)6-7-21-16)23-19(25-20)26-10-12-28-13-11-26/h3-9,21H,2,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mTOR-mediated p70S6K phosphorylation at Thr389 in human PC3 cells |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50008489
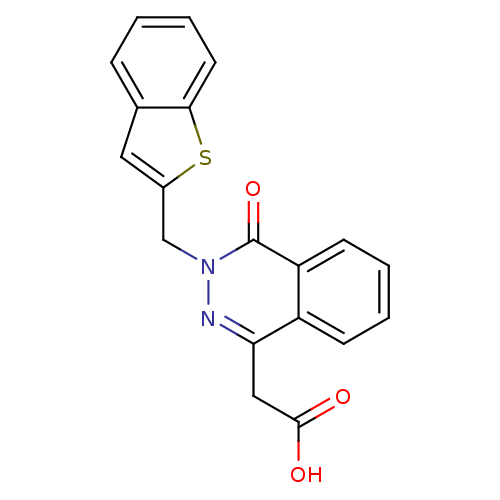
((3-Benzo[b]thiophen-2-ylmethyl-4-oxo-3,4-dihydro-p...)Show InChI InChI=1S/C19H14N2O3S/c22-18(23)10-16-14-6-2-3-7-15(14)19(24)21(20-16)11-13-9-12-5-1-4-8-17(12)25-13/h1-9H,10-11H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of human placental aldose reductase using glyceraldehyde as substrate in presence of NADPH |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50008435
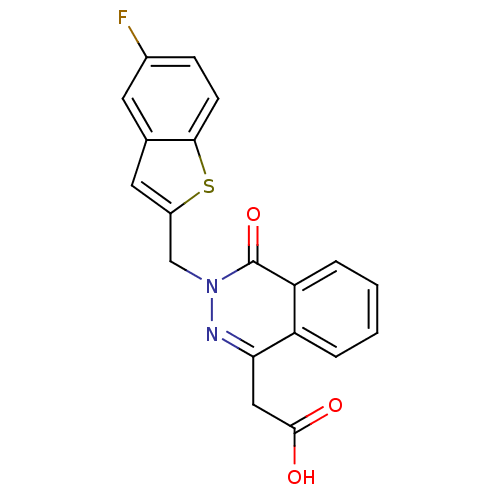
((3-Benzo[b]thiophen-2-ylmethyl-4-oxo-3,4-dihydro-p...)Show SMILES OC(=O)Cc1nn(Cc2cc3cc(F)ccc3s2)c(=O)c2ccccc12 Show InChI InChI=1S/C19H13FN2O3S/c20-12-5-6-17-11(7-12)8-13(26-17)10-22-19(25)15-4-2-1-3-14(15)16(21-22)9-18(23)24/h1-8H,9-10H2,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of human placental aldose reductase using glyceraldehyde as substrate in presence of NADPH |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50276881
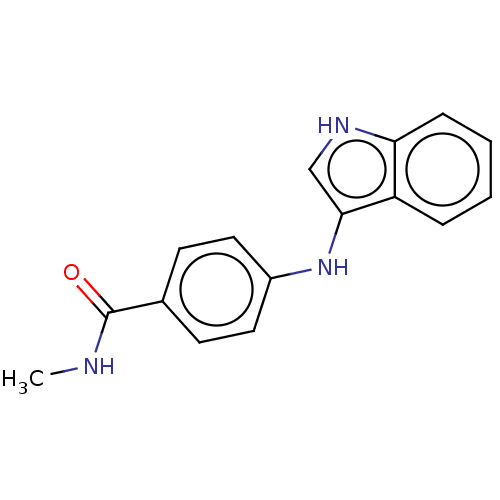
(CHEMBL4160296)Show InChI InChI=1S/C16H15N3O/c1-17-16(20)11-6-8-12(9-7-11)19-15-10-18-14-5-3-2-4-13(14)15/h2-10,18-19H,1H3,(H,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM126720

(US8778932, 31)Show SMILES CC1=NN=C(c2cc3c(cccc3s2)C(F)(F)F)c2cc3oc(=O)[nH]c3cc2C1 |t:1,3| Show InChI InChI=1S/C20H12F3N3O2S/c1-9-5-10-6-14-15(28-19(27)24-14)7-11(10)18(26-25-9)17-8-12-13(20(21,22)23)3-2-4-16(12)29-17/h2-4,6-8H,5H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Antagonist activity at human GABA-A alpha5 receptor expressed in HEK293 cell membranes assessed as inhibition of GABA-induced response preincubated f... |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315213

(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50362405

(CHEMBL1940139)Show InChI InChI=1S/C15H17N9O/c1-10-17-2-3-24(10)15-21-12(11-8-18-13(16)19-9-11)20-14(22-15)23-4-6-25-7-5-23/h2-3,8-9H,4-7H2,1H3,(H2,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM126720

(US8778932, 31)Show SMILES CC1=NN=C(c2cc3c(cccc3s2)C(F)(F)F)c2cc3oc(=O)[nH]c3cc2C1 |t:1,3| Show InChI InChI=1S/C20H12F3N3O2S/c1-9-5-10-6-14-15(28-19(27)24-14)7-11(10)18(26-25-9)17-8-12-13(20(21,22)23)3-2-4-16(12)29-17/h2-4,6-8H,5H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 479 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Antagonist activity at human GABA-A alpha1 receptor expressed in HEK293 cell membranes assessed as inhibition of GABA-induced response preincubated f... |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50362407

(CHEMBL1940141)Show SMILES CCc1cc(nn1-c1nc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C21H20F3N7O/c1-2-13-12-17(21(22,23)24)29-31(13)20-27-18(15-4-3-5-16-14(15)6-7-25-16)26-19(28-20)30-8-10-32-11-9-30/h3-7,12,25H,2,8-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mTOR-mediated p70S6K phosphorylation at Thr389 in human PC3 cells |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50362403
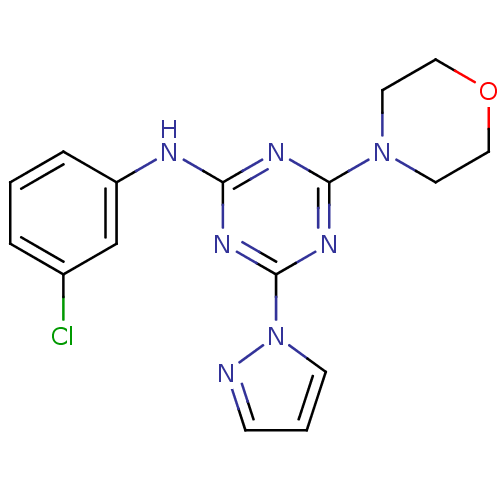
(CHEMBL1940137)Show InChI InChI=1S/C16H16ClN7O/c17-12-3-1-4-13(11-12)19-14-20-15(23-7-9-25-10-8-23)22-16(21-14)24-6-2-5-18-24/h1-6,11H,7-10H2,(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50276879
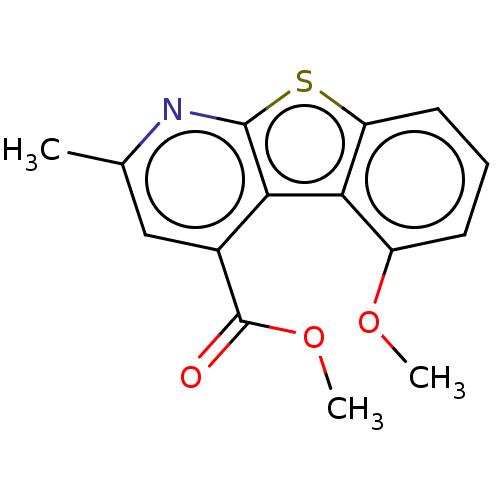
(CHEMBL4177357)Show InChI InChI=1S/C15H13NO3S/c1-8-7-9(15(17)19-3)12-13-10(18-2)5-4-6-11(13)20-14(12)16-8/h4-7H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50362408

(CHEMBL1940142)Show SMILES Cc1nccn1-c1nc(nc(n1)-c1ccc(C)c2[nH]ccc12)N1CCOCC1 Show InChI InChI=1S/C20H21N7O/c1-13-3-4-16(15-5-6-22-17(13)15)18-23-19(26-9-11-28-12-10-26)25-20(24-18)27-8-7-21-14(27)2/h3-8,22H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50276891
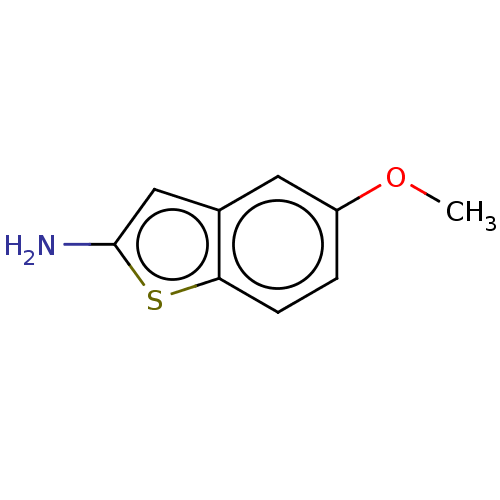
(CHEMBL4176954)Show InChI InChI=1S/C9H9NOS/c1-11-7-2-3-8-6(4-7)5-9(10)12-8/h2-5H,10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50276902
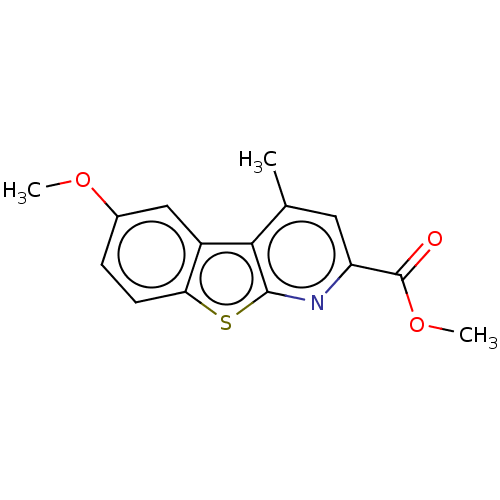
(CHEMBL4169090)Show InChI InChI=1S/C15H13NO3S/c1-8-6-11(15(17)19-3)16-14-13(8)10-7-9(18-2)4-5-12(10)20-14/h4-7H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50362403

(CHEMBL1940137)Show InChI InChI=1S/C16H16ClN7O/c17-12-3-1-4-13(11-12)19-14-20-15(23-7-9-25-10-8-23)22-16(21-14)24-6-2-5-18-24/h1-6,11H,7-10H2,(H,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50362407

(CHEMBL1940141)Show SMILES CCc1cc(nn1-c1nc(nc(n1)-c1cccc2[nH]ccc12)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C21H20F3N7O/c1-2-13-12-17(21(22,23)24)29-31(13)20-27-18(15-4-3-5-16-14(15)6-7-25-16)26-19(28-20)30-8-10-32-11-9-30/h3-7,12,25H,2,8-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 22: 1009-13 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.001
BindingDB Entry DOI: 10.7270/Q2T15439 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50276889
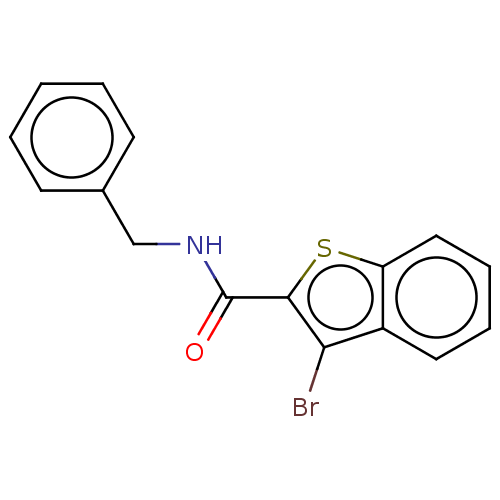
(CHEMBL4170011)Show InChI InChI=1S/C16H12BrNOS/c17-14-12-8-4-5-9-13(12)20-15(14)16(19)18-10-11-6-2-1-3-7-11/h1-9H,10H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jain University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 138: 1002-1033 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.038
BindingDB Entry DOI: 10.7270/Q2C24ZZC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data