 Found 17 hits with Last Name = 'nagashima' and Initial = 'k'
Found 17 hits with Last Name = 'nagashima' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphodiesterase
(Bos taurus) | BDBM50049055
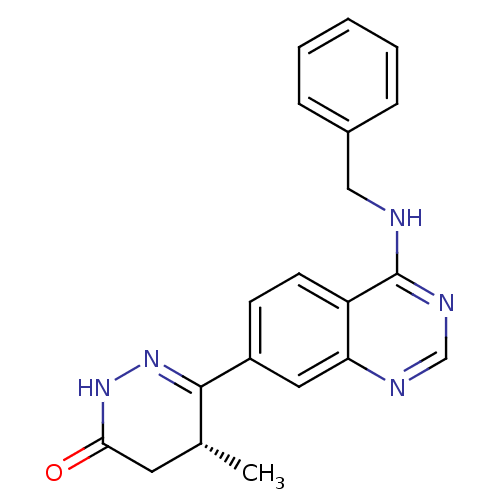
((R)-6-(4-Benzylamino-quinazolin-7-yl)-5-methyl-4,5...)Show SMILES C[C@@H]1CC(=O)NN=C1c1ccc2c(NCc3ccccc3)ncnc2c1 |c:6| Show InChI InChI=1S/C20H19N5O/c1-13-9-18(26)24-25-19(13)15-7-8-16-17(10-15)22-12-23-20(16)21-11-14-5-3-2-4-6-14/h2-8,10,12-13H,9,11H2,1H3,(H,24,26)(H,21,22,23)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakka Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of bovine arterial Phosphodiesterase 3 |
J Med Chem 39: 297-303 (1996)
Article DOI: 10.1021/jm950197j
BindingDB Entry DOI: 10.7270/Q2V125GT |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Bos taurus) | BDBM50049055

((R)-6-(4-Benzylamino-quinazolin-7-yl)-5-methyl-4,5...)Show SMILES C[C@@H]1CC(=O)NN=C1c1ccc2c(NCc3ccccc3)ncnc2c1 |c:6| Show InChI InChI=1S/C20H19N5O/c1-13-9-18(26)24-25-19(13)15-7-8-16-17(10-15)22-12-23-20(16)21-11-14-5-3-2-4-6-14/h2-8,10,12-13H,9,11H2,1H3,(H,24,26)(H,21,22,23)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakka Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of bovine arterial Phosphodiesterase 5 |
J Med Chem 39: 297-303 (1996)
Article DOI: 10.1021/jm950197j
BindingDB Entry DOI: 10.7270/Q2V125GT |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Bos taurus) | BDBM50049053
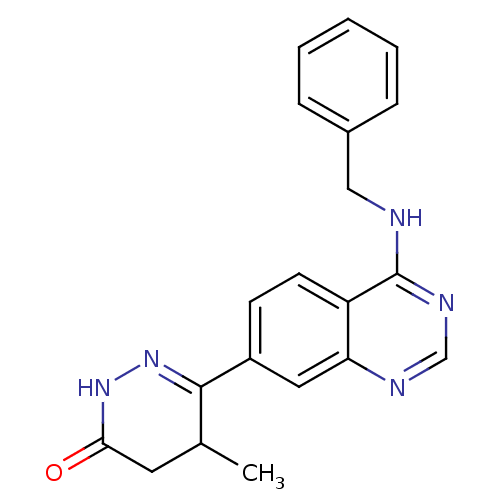
(6-(4-Benzylamino-quinazolin-7-yl)-5-methyl-4,5-dih...)Show SMILES CC1CC(=O)NN=C1c1ccc2c(NCc3ccccc3)ncnc2c1 |c:6| Show InChI InChI=1S/C20H19N5O/c1-13-9-18(26)24-25-19(13)15-7-8-16-17(10-15)22-12-23-20(16)21-11-14-5-3-2-4-6-14/h2-8,10,12-13H,9,11H2,1H3,(H,24,26)(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakka Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of bovine arterial Phosphodiesterase 5 |
J Med Chem 39: 297-303 (1996)
Article DOI: 10.1021/jm950197j
BindingDB Entry DOI: 10.7270/Q2V125GT |
More data for this
Ligand-Target Pair | |
Phosphodiesterase
(Bos taurus) | BDBM50049053

(6-(4-Benzylamino-quinazolin-7-yl)-5-methyl-4,5-dih...)Show SMILES CC1CC(=O)NN=C1c1ccc2c(NCc3ccccc3)ncnc2c1 |c:6| Show InChI InChI=1S/C20H19N5O/c1-13-9-18(26)24-25-19(13)15-7-8-16-17(10-15)22-12-23-20(16)21-11-14-5-3-2-4-6-14/h2-8,10,12-13H,9,11H2,1H3,(H,24,26)(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakka Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of bovine arterial Phosphodiesterase 3 |
J Med Chem 39: 297-303 (1996)
Article DOI: 10.1021/jm950197j
BindingDB Entry DOI: 10.7270/Q2V125GT |
More data for this
Ligand-Target Pair | |
Phosphodiesterase
(Bos taurus) | BDBM50369127
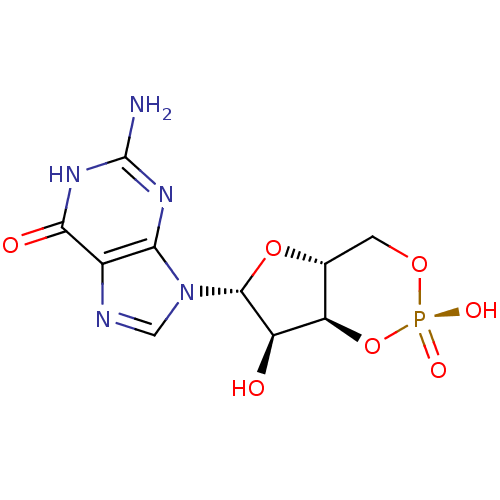
(CHEMBL395336)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@@H]2CO[P@](O)(=O)O[C@H]2[C@H]1O |r| Show InChI InChI=1S/C10H12N5O7P/c11-10-13-7-4(8(17)14-10)12-2-15(7)9-5(16)6-3(21-9)1-20-23(18,19)22-6/h2-3,5-6,9,16H,1H2,(H,18,19)(H3,11,13,14,17)/t3-,5-,6-,9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakka Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of bovine arterial Phosphodiesterase 3 |
J Med Chem 39: 297-303 (1996)
Article DOI: 10.1021/jm950197j
BindingDB Entry DOI: 10.7270/Q2V125GT |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Bos taurus) | BDBM50049054
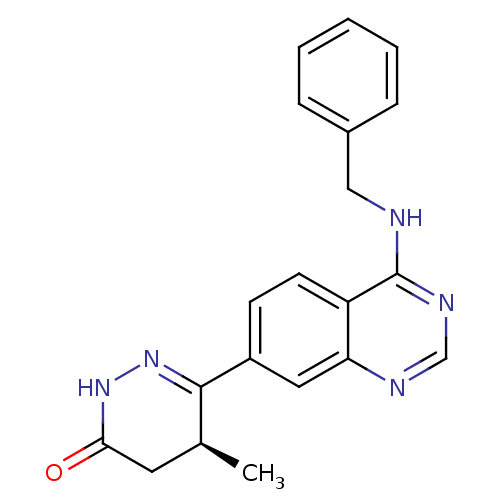
((S)-6-(4-Benzylamino-quinazolin-7-yl)-5-methyl-4,5...)Show SMILES C[C@H]1CC(=O)NN=C1c1ccc2c(NCc3ccccc3)ncnc2c1 |c:6| Show InChI InChI=1S/C20H19N5O/c1-13-9-18(26)24-25-19(13)15-7-8-16-17(10-15)22-12-23-20(16)21-11-14-5-3-2-4-6-14/h2-8,10,12-13H,9,11H2,1H3,(H,24,26)(H,21,22,23)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakka Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of bovine arterial Phosphodiesterase 5 |
J Med Chem 39: 297-303 (1996)
Article DOI: 10.1021/jm950197j
BindingDB Entry DOI: 10.7270/Q2V125GT |
More data for this
Ligand-Target Pair | |
Phosphodiesterase
(Bos taurus) | BDBM15296
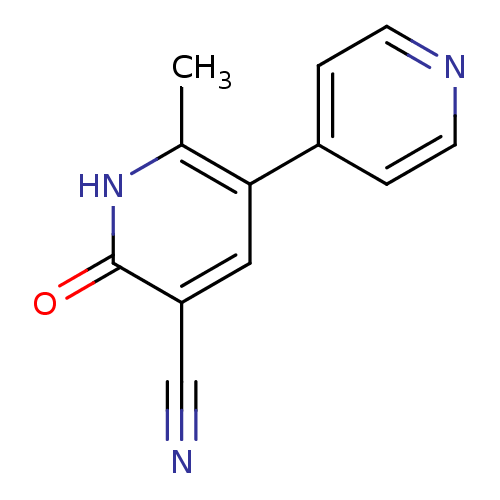
(6-methyl-2-oxo-5-(pyridin-4-yl)-1,2-dihydropyridin...)Show InChI InChI=1S/C12H9N3O/c1-8-11(9-2-4-14-5-3-9)6-10(7-13)12(16)15-8/h2-6H,1H3,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakka Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of bovine arterial Phosphodiesterase 3 |
J Med Chem 39: 297-303 (1996)
Article DOI: 10.1021/jm950197j
BindingDB Entry DOI: 10.7270/Q2V125GT |
More data for this
Ligand-Target Pair | |
Phosphodiesterase
(Bos taurus) | BDBM50049054

((S)-6-(4-Benzylamino-quinazolin-7-yl)-5-methyl-4,5...)Show SMILES C[C@H]1CC(=O)NN=C1c1ccc2c(NCc3ccccc3)ncnc2c1 |c:6| Show InChI InChI=1S/C20H19N5O/c1-13-9-18(26)24-25-19(13)15-7-8-16-17(10-15)22-12-23-20(16)21-11-14-5-3-2-4-6-14/h2-8,10,12-13H,9,11H2,1H3,(H,24,26)(H,21,22,23)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakka Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of bovine arterial Phosphodiesterase 3 |
J Med Chem 39: 297-303 (1996)
Article DOI: 10.1021/jm950197j
BindingDB Entry DOI: 10.7270/Q2V125GT |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Bos taurus) | BDBM15296

(6-methyl-2-oxo-5-(pyridin-4-yl)-1,2-dihydropyridin...)Show InChI InChI=1S/C12H9N3O/c1-8-11(9-2-4-14-5-3-9)6-10(7-13)12(16)15-8/h2-6H,1H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakka Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of bovine arterial Phosphodiesterase 5 |
J Med Chem 39: 297-303 (1996)
Article DOI: 10.1021/jm950197j
BindingDB Entry DOI: 10.7270/Q2V125GT |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Bos taurus) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakka Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of bovine arterial Phosphodiesterase 5 |
J Med Chem 39: 297-303 (1996)
Article DOI: 10.1021/jm950197j
BindingDB Entry DOI: 10.7270/Q2V125GT |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM26198

(4-tert-butyl-15-fluoro-3,5,10-triazatetracyclo[11....)Show SMILES CC(C)(C)c1nc2c([nH]1)c1ccc(F)cc1c1c2cc[nH]c1=O Show InChI InChI=1S/C18H16FN3O/c1-18(2,3)17-21-14-10-5-4-9(19)8-12(10)13-11(15(14)22-17)6-7-20-16(13)23/h4-8H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Enzyme potency (PDK1 EC50)was determined using recombinant, purified full-length human PDK1 enzyme and AKT-Thr-308-tide as substrate. |
J Biol Chem 286: 6433-48 (2011)
Article DOI: 10.1074/jbc.M110.156463
BindingDB Entry DOI: 10.7270/Q21C1VGQ |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM17051

(BX-795 | BX-795, 3 | N-(3-{[5-iodo-4-({3-[(thiophe...)Show SMILES Ic1cnc(Nc2cccc(NC(=O)N3CCCC3)c2)nc1NCCCNC(=O)c1cccs1 Show InChI InChI=1S/C23H26IN7O2S/c24-18-15-27-22(30-20(18)25-9-5-10-26-21(32)19-8-4-13-34-19)28-16-6-3-7-17(14-16)29-23(33)31-11-1-2-12-31/h3-4,6-8,13-15H,1-2,5,9-12H2,(H,26,32)(H,29,33)(H2,25,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Enzyme potency (PDK1 EC50)was determined using recombinant, purified full-length human PDK1 enzyme and AKT-Thr-308-tide as substrate. |
J Biol Chem 286: 6433-48 (2011)
Article DOI: 10.1074/jbc.M110.156463
BindingDB Entry DOI: 10.7270/Q21C1VGQ |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM92406
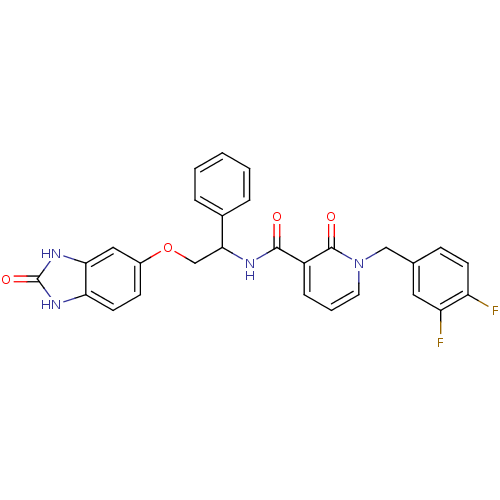
(PDK1 inhibitor, 7)Show SMILES Fc1ccc(Cn2cccc(C(=O)NC(COc3ccc4[nH]c(=O)[nH]c4c3)c3ccccc3)c2=O)cc1F Show InChI InChI=1S/C28H20F2N4O4/c29-21-10-8-17(13-22(21)30)15-34-12-4-7-20(27(34)36)26(35)31-25(18-5-2-1-3-6-18)16-38-19-9-11-23-24(14-19)33-28(37)32-23/h1-14,25H,15-16H2,(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Enzyme potency (PDK1 EC50)was determined using recombinant, purified full-length human PDK1 enzyme and AKT-Thr-308-tide as substrate. |
J Biol Chem 286: 6433-48 (2011)
Article DOI: 10.1074/jbc.M110.156463
BindingDB Entry DOI: 10.7270/Q21C1VGQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM92405
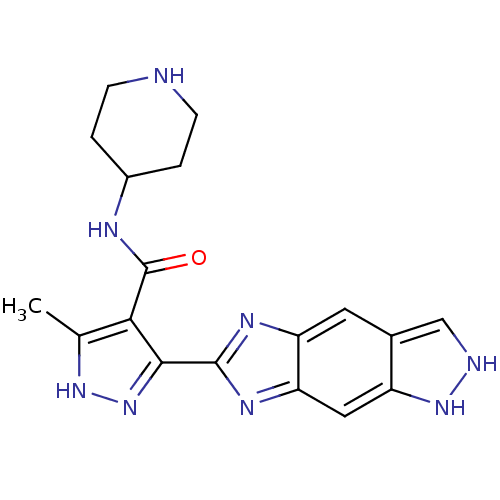
(PDK1 inhibitor, 6)Show SMILES Cc1[nH]nc(-c2nc3cc4c[nH][nH]c4cc3n2)c1C(=O)NC1CCNCC1 Show InChI InChI=1S/C18H20N8O/c1-9-15(18(27)21-11-2-4-19-5-3-11)16(26-24-9)17-22-13-6-10-8-20-25-12(10)7-14(13)23-17/h6-8,11,19-20,25H,2-5H2,1H3,(H,21,27)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Enzyme potency (PDK1 EC50)was determined using recombinant, purified full-length human PDK1 enzyme and AKT-Thr-308-tide as substrate. |
J Biol Chem 286: 6433-48 (2011)
Article DOI: 10.1074/jbc.M110.156463
BindingDB Entry DOI: 10.7270/Q21C1VGQ |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM92404
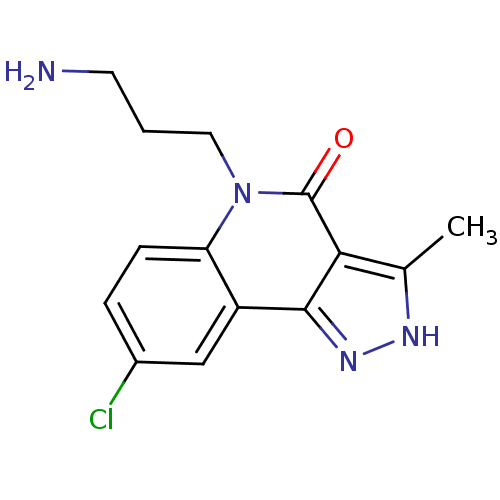
(CHEMBL250843 | PDK1 inhibitor, 5)Show InChI InChI=1S/C14H15ClN4O/c1-8-12-13(18-17-8)10-7-9(15)3-4-11(10)19(14(12)20)6-2-5-16/h3-4,7H,2,5-6,16H2,1H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Enzyme potency (PDK1 EC50)was determined using recombinant, purified full-length human PDK1 enzyme and AKT-Thr-308-tide as substrate. |
J Biol Chem 286: 6433-48 (2011)
Article DOI: 10.1074/jbc.M110.156463
BindingDB Entry DOI: 10.7270/Q21C1VGQ |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM92403
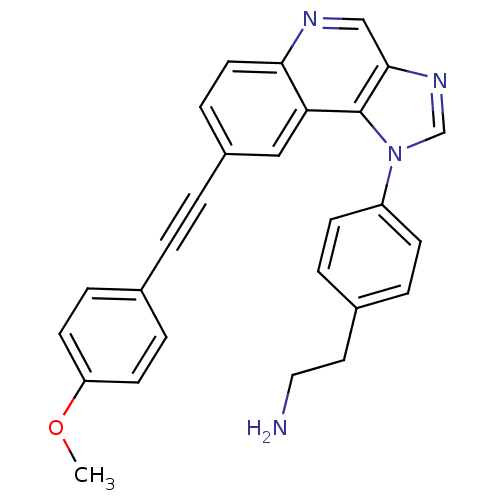
(PDK1 inhibitor, 4)Show SMILES COc1ccc(cc1)C#Cc1ccc2ncc3ncn(-c4ccc(CCN)cc4)c3c2c1 Show InChI InChI=1S/C27H22N4O/c1-32-23-11-6-19(7-12-23)2-3-21-8-13-25-24(16-21)27-26(17-29-25)30-18-31(27)22-9-4-20(5-10-22)14-15-28/h4-13,16-18H,14-15,28H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Enzyme potency (PDK1 EC50)was determined using recombinant, purified full-length human PDK1 enzyme and AKT-Thr-308-tide as substrate. |
J Biol Chem 286: 6433-48 (2011)
Article DOI: 10.1074/jbc.M110.156463
BindingDB Entry DOI: 10.7270/Q21C1VGQ |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM92402
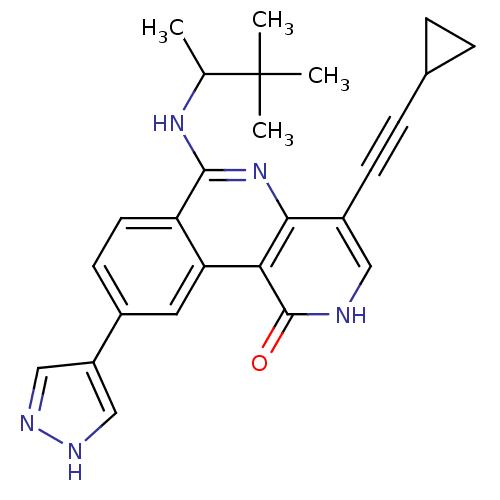
(PDK1 inhibitor, 2)Show SMILES CC(Nc1nc2c(c[nH]c(=O)c2c2cc(ccc12)-c1cn[nH]c1)C#CC1CC1)C(C)(C)C Show InChI InChI=1S/C26H27N5O/c1-15(26(2,3)4)30-24-20-10-9-17(19-13-28-29-14-19)11-21(20)22-23(31-24)18(12-27-25(22)32)8-7-16-5-6-16/h9-16H,5-6H2,1-4H3,(H,27,32)(H,28,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Enzyme potency (PDK1 EC50)was determined using recombinant, purified full-length human PDK1 enzyme and AKT-Thr-308-tide as substrate. |
J Biol Chem 286: 6433-48 (2011)
Article DOI: 10.1074/jbc.M110.156463
BindingDB Entry DOI: 10.7270/Q21C1VGQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data