

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198665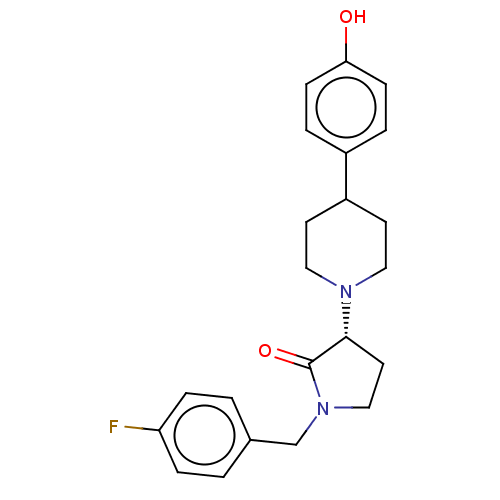 (US9221796, 2b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198694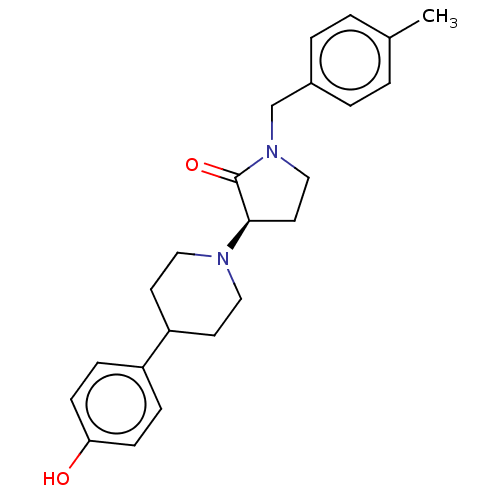 (US9221796, 23b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198728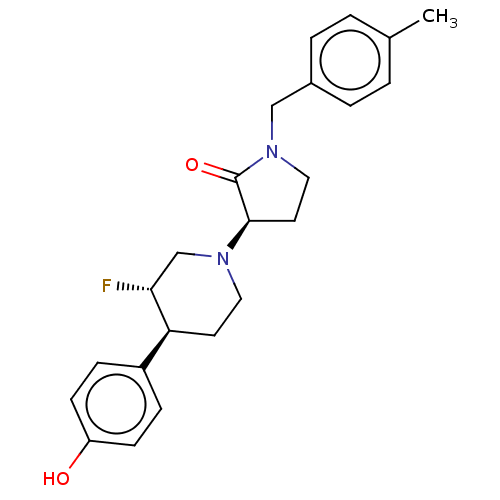 (US9221796, 46, P-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198726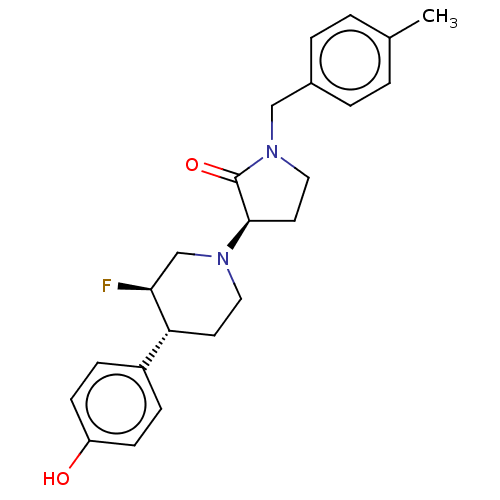 (US9221796, 46, P-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50330324 (CHEMBL4170867) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to GluN2B receptor in human cortex | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50330409 (CHEMBL4168402) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50330410 (CHEMBL4161899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198735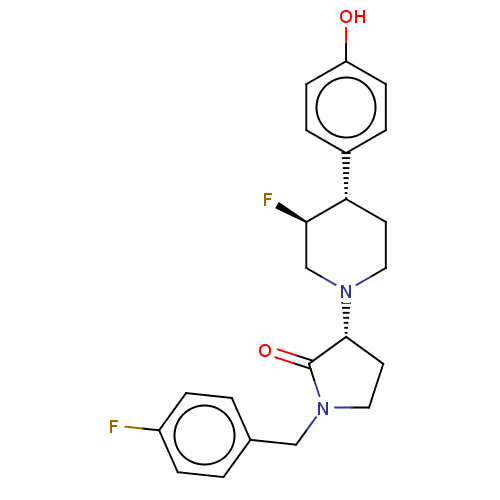 (US9221796, 48, P-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM198665 (US9221796, 2b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to GluN2B receptor in human cortex | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Negative allosteric modulation of human GluN2B receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate/glycine-induced chann... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM198694 (US9221796, 23b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50307794 (2-(4-isobutylphenyl)-N-(3-methylpyridin-2-yl)propa...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Umeň University | Assay Description Frozen (−80┬░C) brains (minus cerebella) from adult Wistar or Sprague-Dawley rats were thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7.... | J Enzyme Inhib Med Chem 28: 172-82 (2013) Article DOI: 10.3109/14756366.2011.643304 BindingDB Entry DOI: 10.7270/Q2BG2MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM198665 (US9221796, 2b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50307794 (2-(4-isobutylphenyl)-N-(3-methylpyridin-2-yl)propa...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Umeň University | Assay Description Frozen (−80┬░C) brains (minus cerebella) from adult Wistar or Sprague-Dawley rats were thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7.... | J Enzyme Inhib Med Chem 28: 172-82 (2013) Article DOI: 10.3109/14756366.2011.643304 BindingDB Entry DOI: 10.7270/Q2BG2MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM237307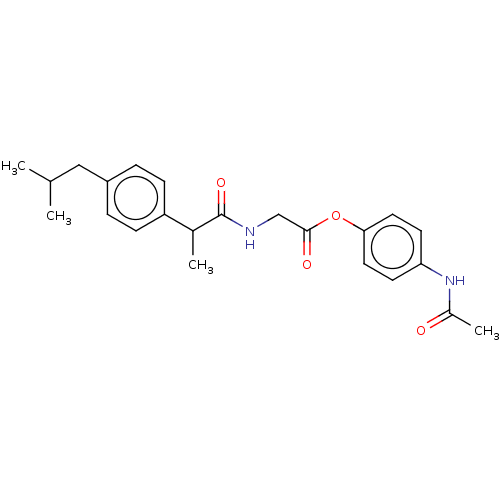 (4-Acetamidophenyl 2-(2-(4-isobutylphenyl)propanami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Umeň University | Assay Description Frozen (−80┬░C) brains (minus cerebella) from adult Wistar or Sprague-Dawley rats were thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7.... | J Enzyme Inhib Med Chem 28: 172-82 (2013) Article DOI: 10.3109/14756366.2011.643304 BindingDB Entry DOI: 10.7270/Q2BG2MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM237308 (ibu-am14 (9)) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Umeň University | Assay Description Frozen (−80┬░C) brains (minus cerebella) from adult Wistar or Sprague-Dawley rats were thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7.... | J Enzyme Inhib Med Chem 28: 172-82 (2013) Article DOI: 10.3109/14756366.2011.643304 BindingDB Entry DOI: 10.7270/Q2BG2MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM198735 (US9221796, 48, P-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50330324 (CHEMBL4170867) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM237310 (2-(4-Isobutylphenyl)-N-(3-(3-methylpyridin-2-ylami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Umeň University | Assay Description Frozen (−80┬░C) brains (minus cerebella) from adult Wistar or Sprague-Dawley rats were thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7.... | J Enzyme Inhib Med Chem 28: 172-82 (2013) Article DOI: 10.3109/14756366.2011.643304 BindingDB Entry DOI: 10.7270/Q2BG2MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using BFC/BZR as substrate | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2B6 in human liver microsomes | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50330409 (CHEMBL4168402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM198726 (US9221796, 46, P-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM237312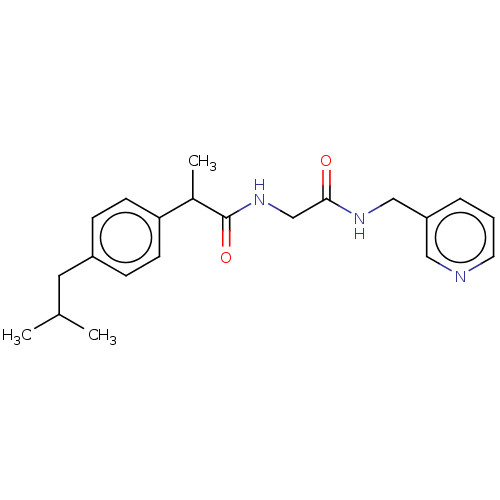 (2-(4-Isobutylphenyl)-N-(2-oxo-2-(pyridin-3-ylmethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Umeň University | Assay Description Frozen (−80┬░C) brains (minus cerebella) from adult Wistar or Sprague-Dawley rats were thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7.... | J Enzyme Inhib Med Chem 28: 172-82 (2013) Article DOI: 10.3109/14756366.2011.643304 BindingDB Entry DOI: 10.7270/Q2BG2MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM237311 (2-(4-Isobutylphenyl)-N-(2-oxo-2-(pyridin-2- ylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Umeň University | Assay Description Frozen (−80┬░C) brains (minus cerebella) from adult Wistar or Sprague-Dawley rats were thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7.... | J Enzyme Inhib Med Chem 28: 172-82 (2013) Article DOI: 10.3109/14756366.2011.643304 BindingDB Entry DOI: 10.7270/Q2BG2MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM237306 (3-Methylpyridin-2-yl 2-(4-isobutylphenyl)propanoat...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Umeň University | Assay Description Frozen (−80┬░C) brains (minus cerebella) from adult Wistar or Sprague-Dawley rats were thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7.... | J Enzyme Inhib Med Chem 28: 172-82 (2013) Article DOI: 10.3109/14756366.2011.643304 BindingDB Entry DOI: 10.7270/Q2BG2MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50009859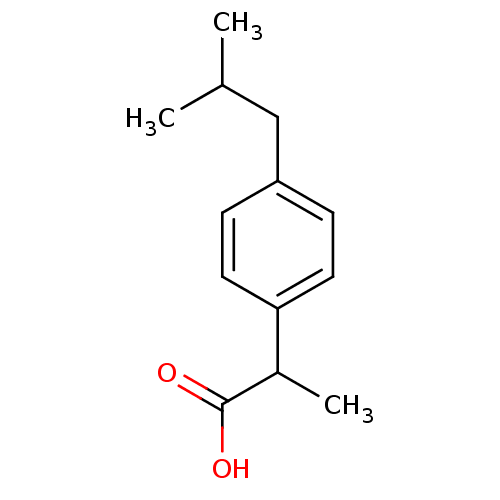 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.34E+5 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Umeň University | Assay Description Frozen (−80┬░C) brains (minus cerebella) from adult Wistar or Sprague-Dawley rats were thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7.... | J Enzyme Inhib Med Chem 28: 172-82 (2013) Article DOI: 10.3109/14756366.2011.643304 BindingDB Entry DOI: 10.7270/Q2BG2MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM237309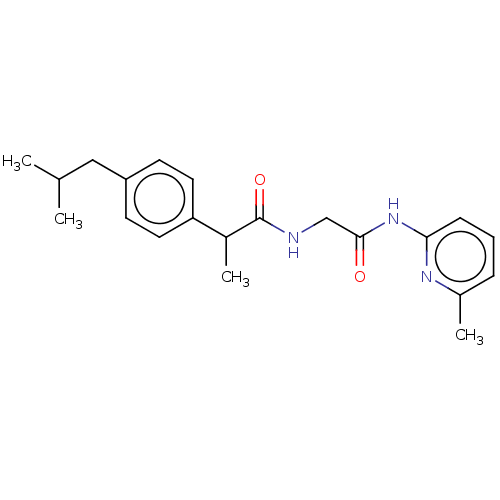 (2-(4-Isobutylphenyl)-N-(2-(6-methylpyridin-2-ylami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Umeň University | Assay Description Frozen (−80┬░C) brains (minus cerebella) from adult Wistar or Sprague-Dawley rats were thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7.... | J Enzyme Inhib Med Chem 28: 172-82 (2013) Article DOI: 10.3109/14756366.2011.643304 BindingDB Entry DOI: 10.7270/Q2BG2MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50009859 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.63E+5 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Umeň University | Assay Description Frozen (−80┬░C) brains (minus cerebella) from adult Wistar or Sprague-Dawley rats were thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7.... | J Enzyme Inhib Med Chem 28: 172-82 (2013) Article DOI: 10.3109/14756366.2011.643304 BindingDB Entry DOI: 10.7270/Q2BG2MWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||