 Found 49 hits with Last Name = 'nakagawa' and Initial = 'r'
Found 49 hits with Last Name = 'nakagawa' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50215149

(CHEMBL108634)Show SMILES COc1ccc(cc1N)-c1sc(N)nc1-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C19H21N3O4S/c1-23-13-6-5-10(7-12(13)20)18-16(22-19(21)27-18)11-8-14(24-2)17(26-4)15(9-11)25-3/h5-9H,20H2,1-4H3,(H2,21,22) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co. Inc.
Curated by ChEMBL
| Assay Description
Tubulin polymerization inhibitory activity using bovine brain tubulin |
Bioorg Med Chem Lett 8: 3153-8 (1998)
BindingDB Entry DOI: 10.7270/Q29K4DD4 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50147362
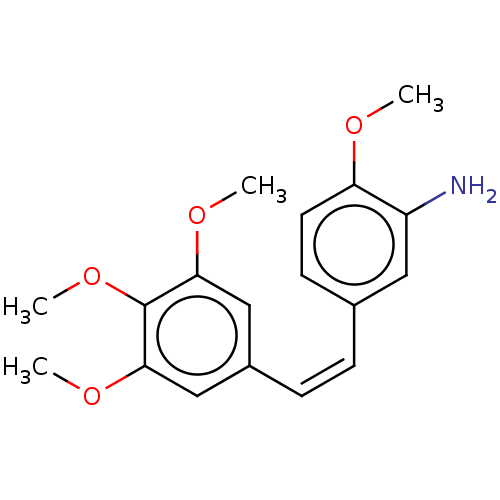
(CHEMBL330407 | ST-3100)Show InChI InChI=1S/C18H21NO4/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11H,19H2,1-4H3/b6-5- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co. Inc.
Curated by ChEMBL
| Assay Description
Tubulin polymerization inhibitory activity using bovine brain tubulin |
Bioorg Med Chem Lett 8: 3153-8 (1998)
BindingDB Entry DOI: 10.7270/Q29K4DD4 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50215145
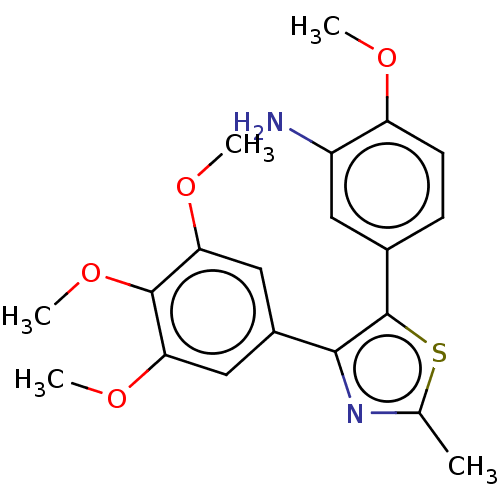
(CHEMBL322529)Show SMILES COc1ccc(cc1N)-c1sc(C)nc1-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C20H22N2O4S/c1-11-22-18(13-9-16(24-3)19(26-5)17(10-13)25-4)20(27-11)12-6-7-15(23-2)14(21)8-12/h6-10H,21H2,1-5H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co. Inc.
Curated by ChEMBL
| Assay Description
Tubulin polymerization inhibitory activity using bovine brain tubulin |
Bioorg Med Chem Lett 8: 3153-8 (1998)
BindingDB Entry DOI: 10.7270/Q29K4DD4 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50215150
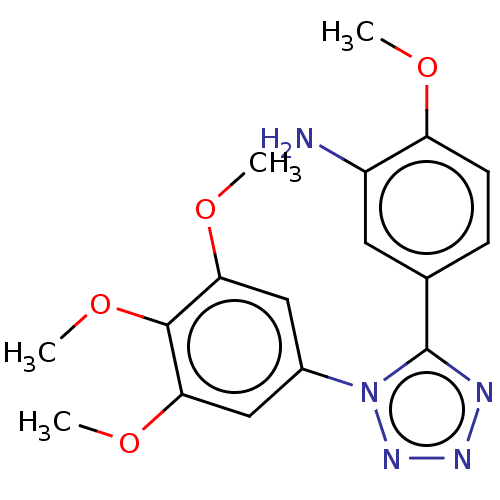
(CHEMBL324397)Show InChI InChI=1S/C17H19N5O4/c1-23-13-6-5-10(7-12(13)18)17-19-20-21-22(17)11-8-14(24-2)16(26-4)15(9-11)25-3/h5-9H,18H2,1-4H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co. Inc.
Curated by ChEMBL
| Assay Description
Tubulin polymerization inhibitory activity using bovine brain tubulin |
Bioorg Med Chem Lett 8: 3153-8 (1998)
BindingDB Entry DOI: 10.7270/Q29K4DD4 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2
(Drosophila melanogaster) | BDBM50081427
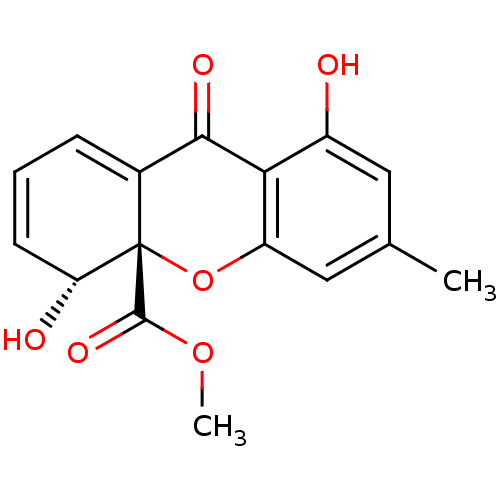
((4R,4aS)-4,8-Dihydroxy-6-methyl-9-oxo-4,9-dihydro-...)Show SMILES COC(=O)[C@]12Oc3cc(C)cc(O)c3C(=O)C1=CC=C[C@H]2O |c:18,20| Show InChI InChI=1S/C16H14O6/c1-8-6-10(17)13-11(7-8)22-16(15(20)21-2)9(14(13)19)4-3-5-12(16)18/h3-7,12,17-18H,1-2H3/t12-,16+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against topoisomerase II (Topo II) by using decatenation assay |
Bioorg Med Chem Lett 9: 2653-6 (1999)
BindingDB Entry DOI: 10.7270/Q2X34XZ8 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471733
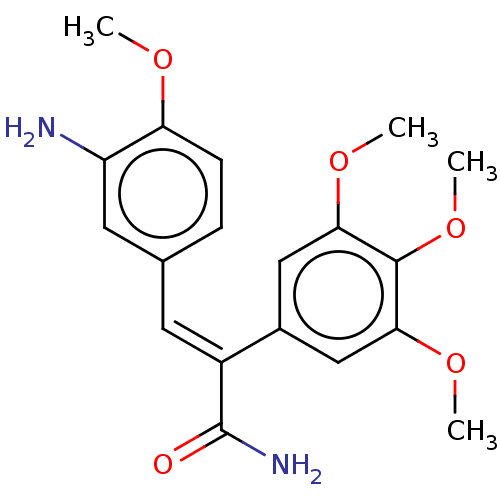
(CHEMBL319183)Show SMILES COc1ccc(\C=C(\C(N)=O)c2cc(OC)c(OC)c(OC)c2)cc1N Show InChI InChI=1S/C19H22N2O5/c1-23-15-6-5-11(8-14(15)20)7-13(19(21)22)12-9-16(24-2)18(26-4)17(10-12)25-3/h5-10H,20H2,1-4H3,(H2,21,22)/b13-7+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471746

(CHEMBL422246)Show InChI InChI=1S/C17H18ClNO3/c1-20-15-9-12(10-16(21-2)17(15)22-3)5-4-11-6-7-13(18)14(19)8-11/h4-10H,19H2,1-3H3/b5-4- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50215153
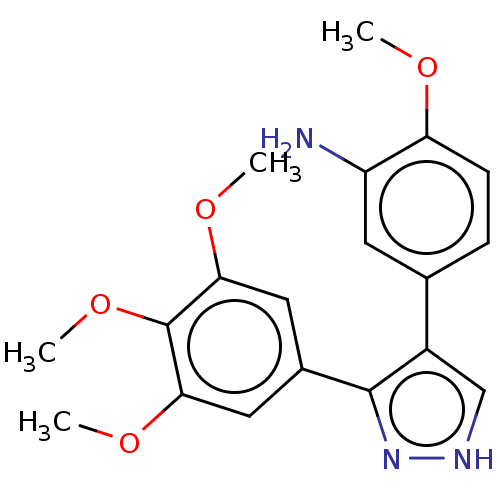
(CHEMBL108621)Show SMILES COc1ccc(cc1N)-c1c[nH]nc1-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C19H21N3O4/c1-23-15-6-5-11(7-14(15)20)13-10-21-22-18(13)12-8-16(24-2)19(26-4)17(9-12)25-3/h5-10H,20H2,1-4H3,(H,21,22) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co. Inc.
Curated by ChEMBL
| Assay Description
Tubulin polymerization inhibitory activity using bovine brain tubulin |
Bioorg Med Chem Lett 8: 3153-8 (1998)
BindingDB Entry DOI: 10.7270/Q29K4DD4 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50215143
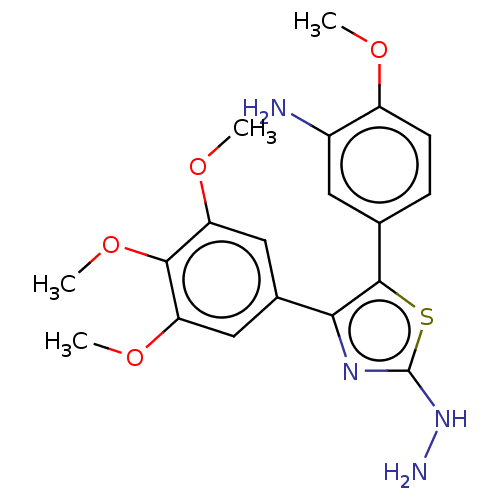
(CHEMBL110326)Show SMILES COc1ccc(cc1N)-c1sc(NN)nc1-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C19H22N4O4S/c1-24-13-6-5-10(7-12(13)20)18-16(22-19(23-21)28-18)11-8-14(25-2)17(27-4)15(9-11)26-3/h5-9H,20-21H2,1-4H3,(H,22,23) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co. Inc.
Curated by ChEMBL
| Assay Description
Tubulin polymerization inhibitory activity using bovine brain tubulin |
Bioorg Med Chem Lett 8: 3153-8 (1998)
BindingDB Entry DOI: 10.7270/Q29K4DD4 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50215151
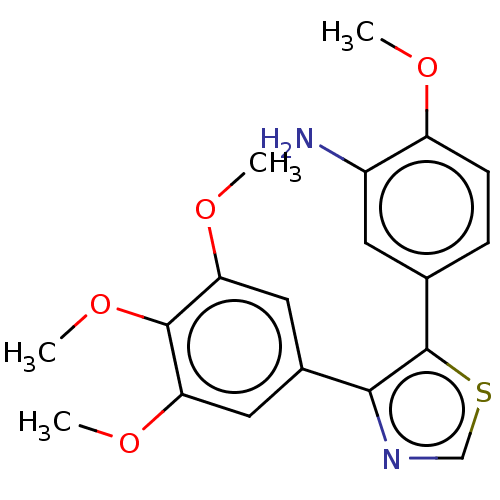
(CHEMBL107416)Show InChI InChI=1S/C19H20N2O4S/c1-22-14-6-5-11(7-13(14)20)19-17(21-10-26-19)12-8-15(23-2)18(25-4)16(9-12)24-3/h5-10H,20H2,1-4H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co. Inc.
Curated by ChEMBL
| Assay Description
Tubulin polymerization inhibitory activity using bovine brain tubulin |
Bioorg Med Chem Lett 8: 3153-8 (1998)
BindingDB Entry DOI: 10.7270/Q29K4DD4 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471747

(CHEMBL102557)Show InChI InChI=1S/C19H20O5/c1-21-16-7-5-13(6-8-16)9-15(12-20)14-10-17(22-2)19(24-4)18(11-14)23-3/h5-12H,1-4H3/b15-9- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471741
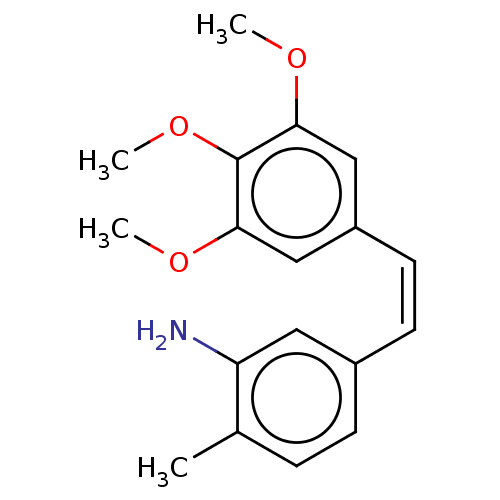
(CHEMBL103333)Show InChI InChI=1S/C18H21NO3/c1-12-5-6-13(9-15(12)19)7-8-14-10-16(20-2)18(22-4)17(11-14)21-3/h5-11H,19H2,1-4H3/b8-7- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50147362

(CHEMBL330407 | ST-3100)Show InChI InChI=1S/C18H21NO4/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11H,19H2,1-4H3/b6-5- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50005480

((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...)Show InChI InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
The compound was evaluated for the anti-tubulin activity. |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50215148

(CHEMBL108518)Show InChI InChI=1S/C18H20N4O4/c1-23-14-6-5-11(7-13(14)19)18-21-20-10-22(18)12-8-15(24-2)17(26-4)16(9-12)25-3/h5-10H,19H2,1-4H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co. Inc.
Curated by ChEMBL
| Assay Description
Tubulin polymerization inhibitory activity using bovine brain tubulin |
Bioorg Med Chem Lett 8: 3153-8 (1998)
BindingDB Entry DOI: 10.7270/Q29K4DD4 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471754

(CHEMBL103062)Show SMILES COc1ccc(\C=C(\C#N)c2cc(OC)c(OC)c(OC)c2)cc1O Show InChI InChI=1S/C19H19NO5/c1-22-16-6-5-12(8-15(16)21)7-14(11-20)13-9-17(23-2)19(25-4)18(10-13)24-3/h5-10,21H,1-4H3/b14-7- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471750

(CHEMBL441739)Show SMILES COc1ccc(\C=C(\C#N)c2cc(OC)c(OC)c(OC)c2)cc1[N+]([O-])=O Show InChI InChI=1S/C19H18N2O6/c1-24-16-6-5-12(8-15(16)21(22)23)7-14(11-20)13-9-17(25-2)19(27-4)18(10-13)26-3/h5-10H,1-4H3/b14-7- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471736

(CHEMBL317013)Show SMILES COc1ccc(cc1N)C(=C/c1cc(OC)c(OC)c(OC)c1)\C#N Show InChI InChI=1S/C19H20N2O4/c1-22-16-6-5-13(10-15(16)21)14(11-20)7-12-8-17(23-2)19(25-4)18(9-12)24-3/h5-10H,21H2,1-4H3/b14-7- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471751
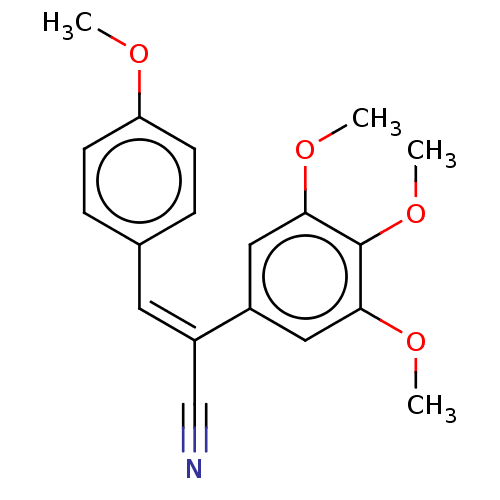
(CHEMBL103856)Show InChI InChI=1S/C19H19NO4/c1-21-16-7-5-13(6-8-16)9-15(12-20)14-10-17(22-2)19(24-4)18(11-14)23-3/h5-11H,1-4H3/b15-9- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471742

(CHEMBL102711)Show SMILES COc1cc(cc(OC)c1OC)C(=C/c1ccc(Cl)c(N)c1)\C#N Show InChI InChI=1S/C18H17ClN2O3/c1-22-16-8-12(9-17(23-2)18(16)24-3)13(10-20)6-11-4-5-14(19)15(21)7-11/h4-9H,21H2,1-3H3/b13-6- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471745
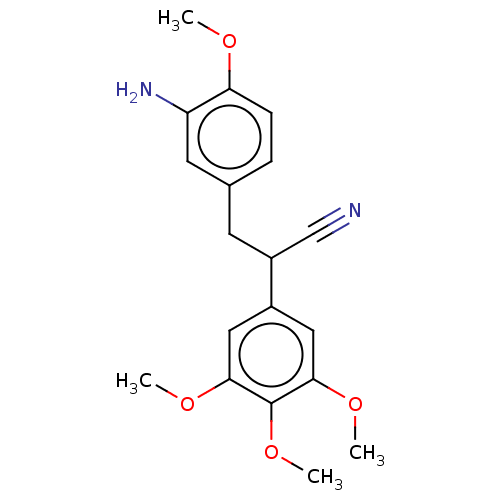
(CHEMBL103329)Show InChI InChI=1S/C19H22N2O4/c1-22-16-6-5-12(8-15(16)21)7-14(11-20)13-9-17(23-2)19(25-4)18(10-13)24-3/h5-6,8-10,14H,7,21H2,1-4H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471752

(CHEMBL102838)Show InChI InChI=1S/C18H18N2O3/c1-21-16-6-4-12(9-15(16)20)8-14(11-19)13-5-7-17(22-2)18(10-13)23-3/h4-10H,20H2,1-3H3/b14-8- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471735
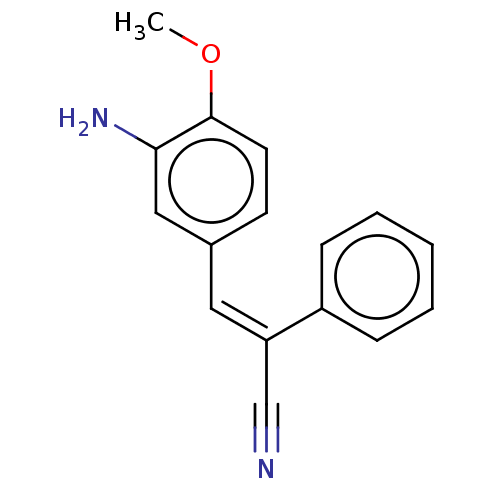
(CHEMBL103288)Show InChI InChI=1S/C16H14N2O/c1-19-16-8-7-12(10-15(16)18)9-14(11-17)13-5-3-2-4-6-13/h2-10H,18H2,1H3/b14-9- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471749
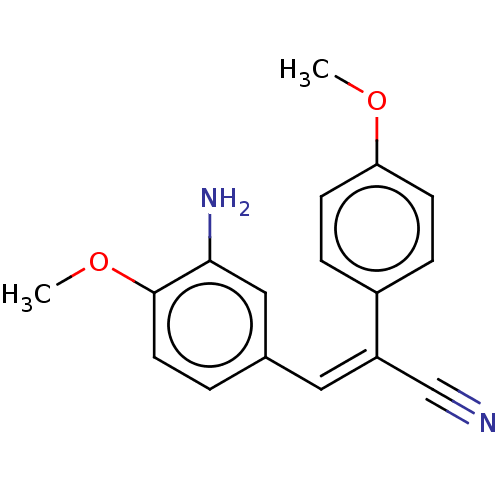
(CHEMBL316750)Show InChI InChI=1S/C17H16N2O2/c1-20-15-6-4-13(5-7-15)14(11-18)9-12-3-8-17(21-2)16(19)10-12/h3-10H,19H2,1-2H3/b14-9- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471732
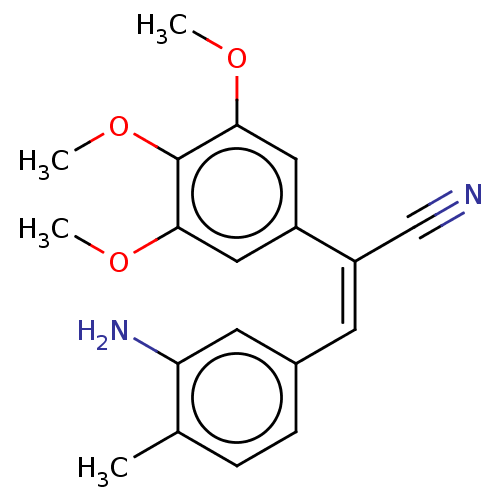
(CHEMBL102944)Show InChI InChI=1S/C19H20N2O3/c1-12-5-6-13(8-16(12)21)7-15(11-20)14-9-17(22-2)19(24-4)18(10-14)23-3/h5-10H,21H2,1-4H3/b15-7- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50215144

(CHEMBL322532)Show SMILES COc1ccc(cc1N)-c1c([nH]nc1[Si](C)(C)C)-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C22H29N3O4Si/c1-26-16-9-8-13(10-15(16)23)19-20(24-25-22(19)30(5,6)7)14-11-17(27-2)21(29-4)18(12-14)28-3/h8-12H,23H2,1-7H3,(H,24,25) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co. Inc.
Curated by ChEMBL
| Assay Description
Tubulin polymerization inhibitory activity using bovine brain tubulin |
Bioorg Med Chem Lett 8: 3153-8 (1998)
BindingDB Entry DOI: 10.7270/Q29K4DD4 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471734
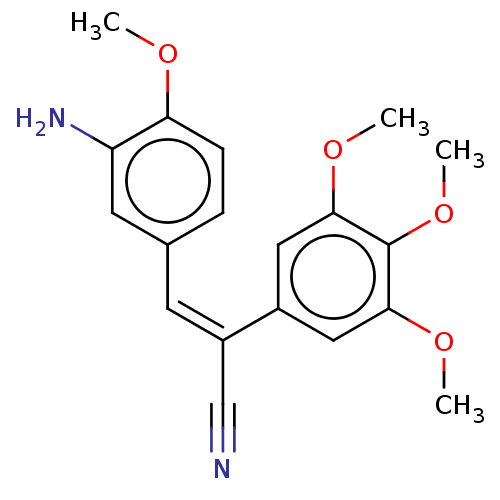
(CHEMBL103582)Show SMILES COc1ccc(\C=C(\C#N)c2cc(OC)c(OC)c(OC)c2)cc1N Show InChI InChI=1S/C19H20N2O4/c1-22-16-6-5-12(8-15(16)21)7-14(11-20)13-9-17(23-2)19(25-4)18(10-13)24-3/h5-10H,21H2,1-4H3/b14-7- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471743

(CHEMBL102949)Show SMILES COc1ccc(\C=C(\C#N)c2cc(OC)c(OC)c(OC)c2)cc1NC(C)=O Show InChI InChI=1S/C21H22N2O5/c1-13(24)23-17-9-14(6-7-18(17)25-2)8-16(12-22)15-10-19(26-3)21(28-5)20(11-15)27-4/h6-11H,1-5H3,(H,23,24)/b16-8- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50215146

(CHEMBL317022)Show SMILES COc1ccc(cc1N)-c1nnc(C)n1-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C19H22N4O4/c1-11-21-22-19(12-6-7-15(24-2)14(20)8-12)23(11)13-9-16(25-3)18(27-5)17(10-13)26-4/h6-10H,20H2,1-5H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co. Inc.
Curated by ChEMBL
| Assay Description
Tubulin polymerization inhibitory activity using bovine brain tubulin |
Bioorg Med Chem Lett 8: 3153-8 (1998)
BindingDB Entry DOI: 10.7270/Q29K4DD4 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50215152
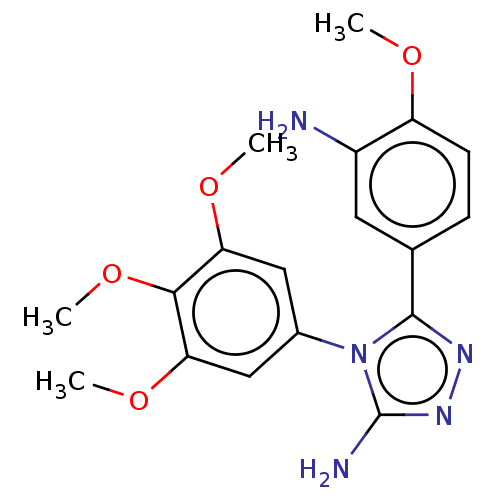
(CHEMBL320117)Show SMILES COc1ccc(cc1N)-c1nnc(N)n1-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C18H21N5O4/c1-24-13-6-5-10(7-12(13)19)17-21-22-18(20)23(17)11-8-14(25-2)16(27-4)15(9-11)26-3/h5-9H,19H2,1-4H3,(H2,20,22) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co. Inc.
Curated by ChEMBL
| Assay Description
Tubulin polymerization inhibitory activity using bovine brain tubulin |
Bioorg Med Chem Lett 8: 3153-8 (1998)
BindingDB Entry DOI: 10.7270/Q29K4DD4 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50215147
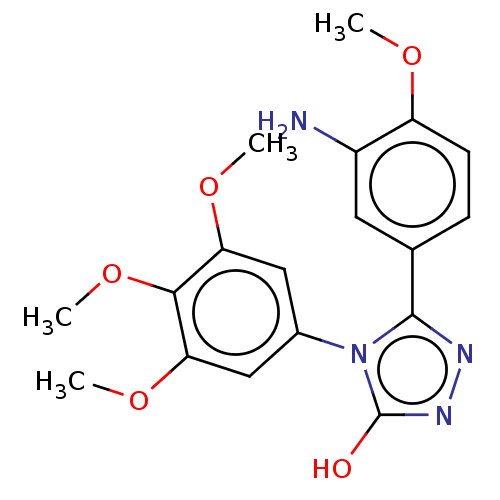
(CHEMBL107424)Show SMILES COc1ccc(cc1N)-c1nnc(O)n1-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C18H20N4O5/c1-24-13-6-5-10(7-12(13)19)17-20-21-18(23)22(17)11-8-14(25-2)16(27-4)15(9-11)26-3/h5-9H,19H2,1-4H3,(H,21,23) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co. Inc.
Curated by ChEMBL
| Assay Description
Tubulin polymerization inhibitory activity using bovine brain tubulin |
Bioorg Med Chem Lett 8: 3153-8 (1998)
BindingDB Entry DOI: 10.7270/Q29K4DD4 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471744
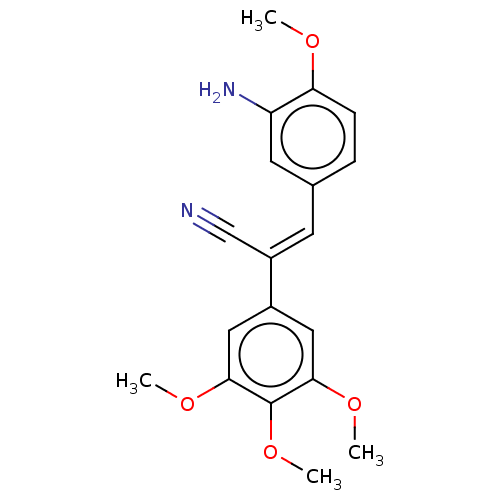
(CHEMBL105075)Show SMILES COc1ccc(\C=C(/C#N)c2cc(OC)c(OC)c(OC)c2)cc1N Show InChI InChI=1S/C19H20N2O4/c1-22-16-6-5-12(8-15(16)21)7-14(11-20)13-9-17(23-2)19(25-4)18(10-13)24-3/h5-10H,21H2,1-4H3/b14-7+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471740

(CHEMBL102702)Show InChI InChI=1S/C19H22O5/c1-21-16-7-5-13(6-8-16)9-15(12-20)14-10-17(22-2)19(24-4)18(11-14)23-3/h5-11,20H,12H2,1-4H3/b15-9- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471738
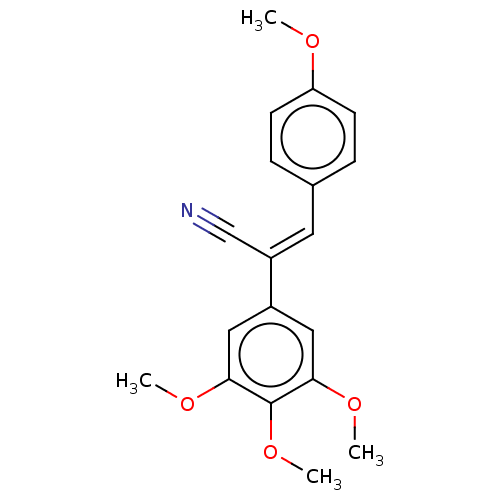
(CHEMBL322446)Show InChI InChI=1S/C19H19NO4/c1-21-16-7-5-13(6-8-16)9-15(12-20)14-10-17(22-2)19(24-4)18(11-14)23-3/h5-11H,1-4H3/b15-9+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471737

(CHEMBL105217)Show SMILES [I-].COc1ccc(\C=C(\C[N+](C)(C)C)c2cc(OC)c(OC)c(OC)c2)cc1 Show InChI InChI=1S/C22H30NO4/c1-23(2,3)15-18(12-16-8-10-19(24-4)11-9-16)17-13-20(25-5)22(27-7)21(14-17)26-6/h8-14H,15H2,1-7H3/q+1/b18-12- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471753
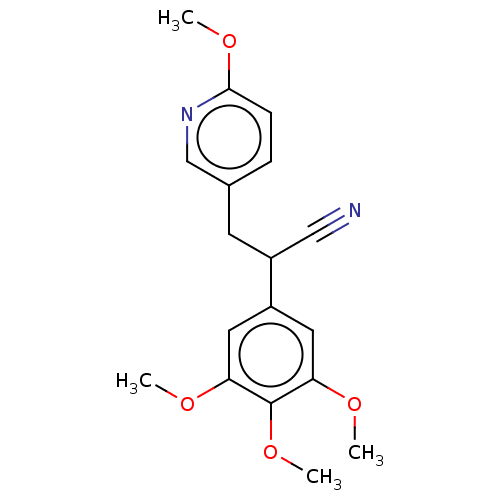
(CHEMBL102235)Show InChI InChI=1S/C18H20N2O4/c1-21-15-8-13(9-16(22-2)18(15)24-4)14(10-19)7-12-5-6-17(23-3)20-11-12/h5-6,8-9,11,14H,7H2,1-4H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471731

(CHEMBL320679)Show InChI InChI=1S/C19H20N2O3/c1-12-5-6-13(8-16(12)21)7-15(11-20)14-9-17(22-2)19(24-4)18(10-14)23-3/h5-10H,21H2,1-4H3/b15-7+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471739
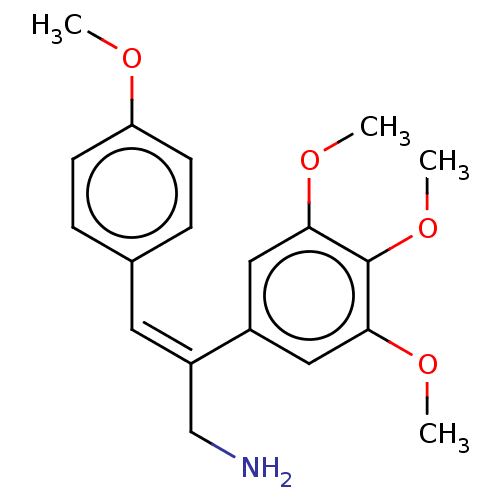
(CHEMBL103174)Show InChI InChI=1S/C19H23NO4/c1-21-16-7-5-13(6-8-16)9-15(12-20)14-10-17(22-2)19(24-4)18(11-14)23-3/h5-11H,12,20H2,1-4H3/b15-9- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471755

(CHEMBL103653)Show InChI InChI=1S/C18H18N2O4/c1-21-15-8-13(9-16(22-2)18(15)24-4)14(10-19)7-12-5-6-17(23-3)20-11-12/h5-9,11H,1-4H3/b14-7- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
Similar to alpha-tubulin isoform 1
(Bos taurus) | BDBM50471748

(CHEMBL322173)Show SMILES COc1ccc(\C=C(/C#N)c2cc(OC)c(OC)c(OC)c2)cc1[N+]([O-])=O Show InChI InChI=1S/C19H18N2O6/c1-24-16-6-5-12(8-15(16)21(22)23)7-14(11-20)13-9-17(25-2)19(27-4)18(10-13)26-3/h5-10H,1-4H3/b14-7+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tubulin polymerization |
J Med Chem 41: 3022-32 (1998)
Article DOI: 10.1021/jm980101w
BindingDB Entry DOI: 10.7270/Q2QV3Q85 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2
(Drosophila melanogaster) | BDBM50081428

((4R,4aS)-4,8-Dihydroxy-6-methyl-9-oxo-4,9-dihydro-...)Show SMILES Cc1cc(O)c2C(=O)C3=CC=C[C@@H](O)[C@]3(Oc2c1)C(=O)NCCO |c:10,t:8| Show InChI InChI=1S/C17H17NO6/c1-9-7-11(20)14-12(8-9)24-17(16(23)18-5-6-19)10(15(14)22)3-2-4-13(17)21/h2-4,7-8,13,19-21H,5-6H2,1H3,(H,18,23)/t13-,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against topoisomerase II (Topo II) by using decatenation assay |
Bioorg Med Chem Lett 9: 2653-6 (1999)
BindingDB Entry DOI: 10.7270/Q2X34XZ8 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2
(Drosophila melanogaster) | BDBM50081429

((4R,4aS)-4,8-Dihydroxy-6-methyl-9-oxo-4,9-dihydro-...)Show SMILES Cc1cc(O)c2C(=O)C3=CC=C[C@@H](O)[C@]3(Oc2c1)C(N)=O |c:10,t:8| Show InChI InChI=1S/C15H13NO5/c1-7-5-9(17)12-10(6-7)21-15(14(16)20)8(13(12)19)3-2-4-11(15)18/h2-6,11,17-18H,1H3,(H2,16,20)/t11-,15+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against topoisomerase II (Topo II) by using decatenation assay |
Bioorg Med Chem Lett 9: 2653-6 (1999)
BindingDB Entry DOI: 10.7270/Q2X34XZ8 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Mus musculus) | BDBM50081429

((4R,4aS)-4,8-Dihydroxy-6-methyl-9-oxo-4,9-dihydro-...)Show SMILES Cc1cc(O)c2C(=O)C3=CC=C[C@@H](O)[C@]3(Oc2c1)C(N)=O |c:10,t:8| Show InChI InChI=1S/C15H13NO5/c1-7-5-9(17)12-10(6-7)21-15(14(16)20)8(13(12)19)3-2-4-11(15)18/h2-6,11,17-18H,1H3,(H2,16,20)/t11-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against topoisomerase I (Topo I) by using relaxation assay |
Bioorg Med Chem Lett 9: 2653-6 (1999)
BindingDB Entry DOI: 10.7270/Q2X34XZ8 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Mus musculus) | BDBM50081428

((4R,4aS)-4,8-Dihydroxy-6-methyl-9-oxo-4,9-dihydro-...)Show SMILES Cc1cc(O)c2C(=O)C3=CC=C[C@@H](O)[C@]3(Oc2c1)C(=O)NCCO |c:10,t:8| Show InChI InChI=1S/C17H17NO6/c1-9-7-11(20)14-12(8-9)24-17(16(23)18-5-6-19)10(15(14)22)3-2-4-13(17)21/h2-4,7-8,13,19-21H,5-6H2,1H3,(H,18,23)/t13-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against topoisomerase I (Topo I) by using relaxation assay |
Bioorg Med Chem Lett 9: 2653-6 (1999)
BindingDB Entry DOI: 10.7270/Q2X34XZ8 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2
(Drosophila melanogaster) | BDBM50081426
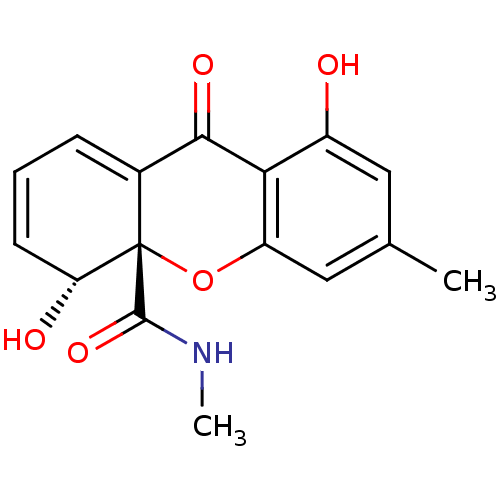
((4R,4aS)-4,8-Dihydroxy-6-methyl-9-oxo-4,9-dihydro-...)Show SMILES CNC(=O)[C@]12Oc3cc(C)cc(O)c3C(=O)C1=CC=C[C@H]2O |c:18,20| Show InChI InChI=1S/C16H15NO5/c1-8-6-10(18)13-11(7-8)22-16(15(21)17-2)9(14(13)20)4-3-5-12(16)19/h3-7,12,18-19H,1-2H3,(H,17,21)/t12-,16+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against topoisomerase II (Topo II) by using decatenation assay |
Bioorg Med Chem Lett 9: 2653-6 (1999)
BindingDB Entry DOI: 10.7270/Q2X34XZ8 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Mus musculus) | BDBM50081427

((4R,4aS)-4,8-Dihydroxy-6-methyl-9-oxo-4,9-dihydro-...)Show SMILES COC(=O)[C@]12Oc3cc(C)cc(O)c3C(=O)C1=CC=C[C@H]2O |c:18,20| Show InChI InChI=1S/C16H14O6/c1-8-6-10(17)13-11(7-8)22-16(15(20)21-2)9(14(13)19)4-3-5-12(16)18/h3-7,12,17-18H,1-2H3/t12-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against topoisomerase I (Topo I) by using relaxation assay |
Bioorg Med Chem Lett 9: 2653-6 (1999)
BindingDB Entry DOI: 10.7270/Q2X34XZ8 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Mus musculus) | BDBM50081426

((4R,4aS)-4,8-Dihydroxy-6-methyl-9-oxo-4,9-dihydro-...)Show SMILES CNC(=O)[C@]12Oc3cc(C)cc(O)c3C(=O)C1=CC=C[C@H]2O |c:18,20| Show InChI InChI=1S/C16H15NO5/c1-8-6-10(18)13-11(7-8)22-16(15(21)17-2)9(14(13)20)4-3-5-12(16)19/h3-7,12,18-19H,1-2H3,(H,17,21)/t12-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against topoisomerase I (Topo I) by using relaxation assay |
Bioorg Med Chem Lett 9: 2653-6 (1999)
BindingDB Entry DOI: 10.7270/Q2X34XZ8 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2
(Drosophila melanogaster) | BDBM50081431
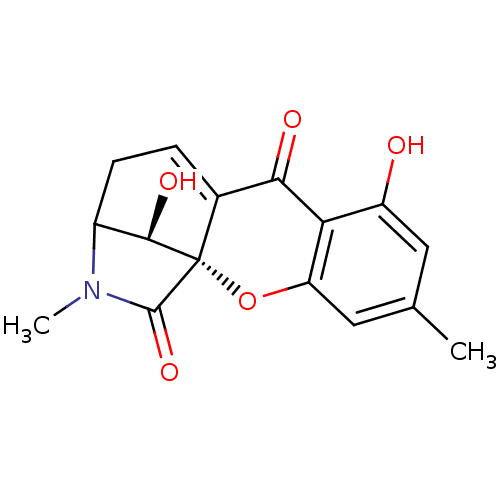
(7,16-dihydroxy-5,14-dimethyl-(1S,13S,16R)-2-oxa-14...)Show SMILES CN1C2CC=C3C(=O)c4c(O)cc(C)cc4O[C@@]3([C@@H]2O)C1=O |t:4,THB:0:1:18:5.4.3,21:20:18:5.4.3| Show InChI InChI=1S/C16H15NO5/c1-7-5-10(18)12-11(6-7)22-16-8(13(12)19)3-4-9(14(16)20)17(2)15(16)21/h3,5-6,9,14,18,20H,4H2,1-2H3/t9?,14-,16+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against topoisomerase II (Topo II) by using decatenation assay |
Bioorg Med Chem Lett 9: 2653-6 (1999)
BindingDB Entry DOI: 10.7270/Q2X34XZ8 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Mus musculus) | BDBM50081431

(7,16-dihydroxy-5,14-dimethyl-(1S,13S,16R)-2-oxa-14...)Show SMILES CN1C2CC=C3C(=O)c4c(O)cc(C)cc4O[C@@]3([C@@H]2O)C1=O |t:4,THB:0:1:18:5.4.3,21:20:18:5.4.3| Show InChI InChI=1S/C16H15NO5/c1-7-5-10(18)12-11(6-7)22-16-8(13(12)19)3-4-9(14(16)20)17(2)15(16)21/h3,5-6,9,14,18,20H,4H2,1-2H3/t9?,14-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against topoisomerase I (Topo I) by using relaxation assay |
Bioorg Med Chem Lett 9: 2653-6 (1999)
BindingDB Entry DOI: 10.7270/Q2X34XZ8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data