 Found 9 hits with Last Name = 'narang' and Initial = 'rk'
Found 9 hits with Last Name = 'narang' and Initial = 'rk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50528546

(CHEMBL4516243)Show SMILES O=S(=O)(CC1CS1)c1ccc(cc1)-n1cc(nn1)-c1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C22H18N4O2S2/c27-30(28,15-20-14-29-20)21-7-5-19(6-8-21)26-13-22(24-25-26)18-3-1-16(2-4-18)17-9-11-23-12-10-17/h1-13,20H,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 catalytic domain (unknown origin) using Ac-PLG-[2-mercapto-4-methylpentanoyl]-LG-OC2H5 as substrate preincubated for 45 to 120 min... |
Eur J Med Chem 180: 486-508 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.043
BindingDB Entry DOI: 10.7270/Q2959N1V |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) by EIA |
Eur J Med Chem 180: 486-508 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.043
BindingDB Entry DOI: 10.7270/Q2959N1V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50528543
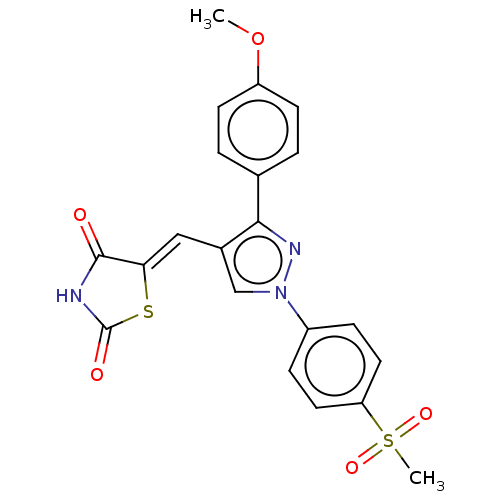
(CHEMBL4540611)Show SMILES COc1ccc(cc1)-c1nn(cc1\C=C1/SC(=O)NC1=O)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C21H17N3O5S2/c1-29-16-7-3-13(4-8-16)19-14(11-18-20(25)22-21(26)30-18)12-24(23-19)15-5-9-17(10-6-15)31(2,27)28/h3-12H,1-2H3,(H,22,25,26)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) by EIA |
Eur J Med Chem 180: 486-508 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.043
BindingDB Entry DOI: 10.7270/Q2959N1V |
More data for this
Ligand-Target Pair | |
Large neutral amino acids transporter small subunit 1
(Homo sapiens (Human)) | BDBM50528545

(CHEMBL4526588)Show InChI InChI=1S/C8H3ClF3N3OS2/c9-6-7(17-18-15-6)14-5-2-1-4(3-13-5)16-8(10,11)12/h1-3H/b14-7- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LAT1 |
Eur J Med Chem 180: 486-508 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.043
BindingDB Entry DOI: 10.7270/Q2959N1V |
More data for this
Ligand-Target Pair | |
Probable maltase-glucoamylase 2
(Homo sapiens) | BDBM50502004
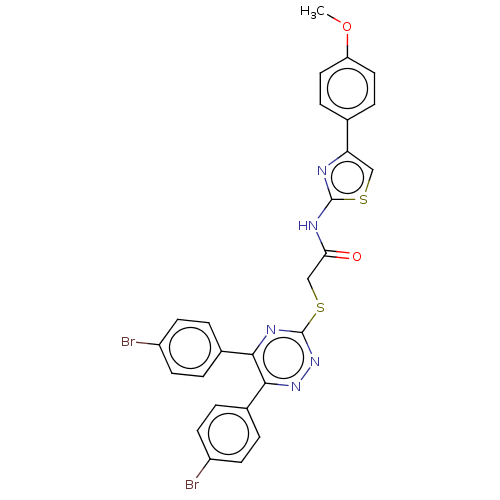
(CHEMBL4453256)Show SMILES COc1ccc(cc1)-c1csc(NC(=O)CSc2nnc(-c3ccc(Br)cc3)c(n2)-c2ccc(Br)cc2)n1 Show InChI InChI=1S/C27H19Br2N5O2S2/c1-36-21-12-6-16(7-13-21)22-14-37-26(30-22)31-23(35)15-38-27-32-24(17-2-8-19(28)9-3-17)25(33-34-27)18-4-10-20(29)11-5-18/h2-14H,15H2,1H3,(H,30,31,35) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University
Curated by ChEMBL
| Assay Description
Inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl alpha-d-glucopyranoside as substrate preincubated for 15 mins followed by substr... |
Eur J Med Chem 180: 486-508 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.043
BindingDB Entry DOI: 10.7270/Q2959N1V |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50000541

((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...)Show InChI InChI=1S/C11H12N2O2S/c1-7(13(15)11(12)14)10-6-8-4-2-3-5-9(8)16-10/h2-7,15H,1H3,(H2,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) |
Eur J Med Chem 180: 486-508 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.043
BindingDB Entry DOI: 10.7270/Q2959N1V |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM152734

(11-bromo-5-[4-(2-fluorophenyl)piperazine-1- carbon...)Show SMILES Fc1ccccc1N1CCN(CC1)C(=O)c1[nH]nc-2c1CSc1sc(Br)cc-21 Show InChI InChI=1S/C19H16BrFN4OS2/c20-15-9-11-16-12(10-27-19(11)28-15)17(23-22-16)18(26)25-7-5-24(6-8-25)14-4-2-1-3-13(14)21/h1-4,9H,5-8,10H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) |
Eur J Med Chem 180: 486-508 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.043
BindingDB Entry DOI: 10.7270/Q2959N1V |
More data for this
Ligand-Target Pair | |
Probable maltase-glucoamylase 2
(Homo sapiens) | BDBM23406

((3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5...)Show SMILES C[C@H]1O[C@H](O[C@@H]2[C@@H](CO)O[C@H](O[C@@H]3[C@@H](CO)OC(O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C=C(CO)[C@@H](O)[C@H](O)[C@H]1O |t:37| Show InChI InChI=1S/C25H43NO18/c1-6-11(26-8-2-7(3-27)12(30)15(33)13(8)31)14(32)19(37)24(40-6)43-22-10(5-29)42-25(20(38)17(22)35)44-21-9(4-28)41-23(39)18(36)16(21)34/h2,6,8-39H,3-5H2,1H3/t6-,8+,9-,10-,11-,12-,13+,14+,15+,16-,17-,18-,19-,20-,21-,22-,23?,24-,25-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University
Curated by ChEMBL
| Assay Description
Inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl alpha-d-glucopyranoside as substrate preincubated for 15 mins followed by substr... |
Eur J Med Chem 180: 486-508 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.043
BindingDB Entry DOI: 10.7270/Q2959N1V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50528544
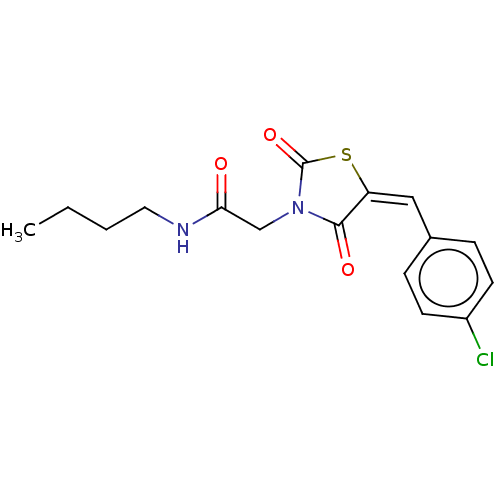
(CHEMBL4587922)Show InChI InChI=1S/C16H17ClN2O3S/c1-2-3-8-18-14(20)10-19-15(21)13(23-16(19)22)9-11-4-6-12(17)7-5-11/h4-7,9H,2-3,8,10H2,1H3,(H,18,20)/b13-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma (unknown origin) |
Eur J Med Chem 180: 486-508 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.043
BindingDB Entry DOI: 10.7270/Q2959N1V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data