 Found 744 hits with Last Name = 'navre' and Initial = 'm'
Found 744 hits with Last Name = 'navre' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070256
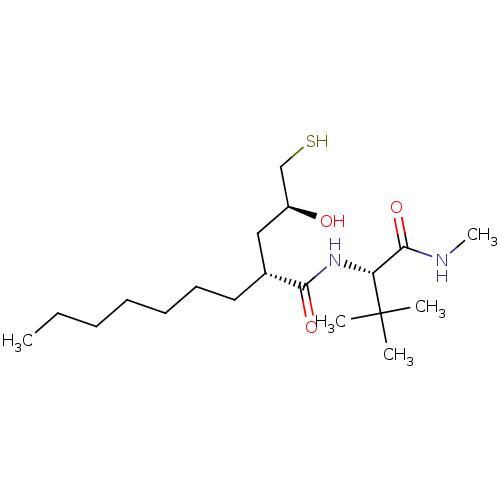
((R)-2-((S)-2-Hydroxy-3-mercapto-propyl)-nonanoic a...)Show SMILES CCCCCCC[C@H](C[C@H](O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C19H38N2O3S/c1-6-7-8-9-10-11-14(12-15(22)13-25)17(23)21-16(18(24)20-5)19(2,3)4/h14-16,22,25H,6-13H2,1-5H3,(H,20,24)(H,21,23)/t14-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-9, gelatinase-B |
Bioorg Med Chem Lett 8: 1163-8 (1999)
BindingDB Entry DOI: 10.7270/Q2348JH9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070257
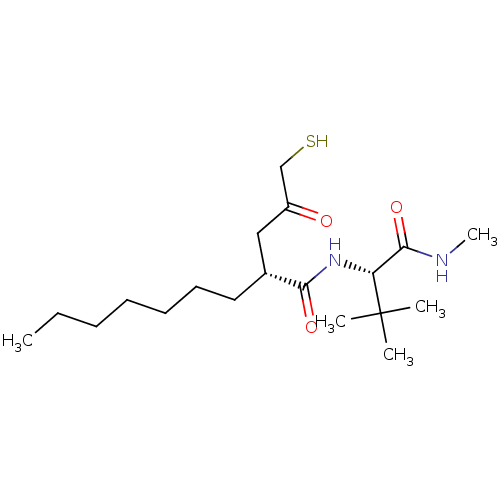
((R)-2-(3-Mercapto-2-oxo-propyl)-nonanoic acid ((S)...)Show SMILES CCCCCCC[C@H](CC(=O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C19H36N2O3S/c1-6-7-8-9-10-11-14(12-15(22)13-25)17(23)21-16(18(24)20-5)19(2,3)4/h14,16,25H,6-13H2,1-5H3,(H,20,24)(H,21,23)/t14-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-9, gelatinase-B |
Bioorg Med Chem Lett 8: 1163-8 (1999)
BindingDB Entry DOI: 10.7270/Q2348JH9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070228

(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-2,2-dimet...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C18H34N2O3S/c1-6-7-8-9-10-11-13(14(21)12-24)16(22)20-15(17(23)19-5)18(2,3)4/h13,15,24H,6-12H2,1-5H3,(H,19,23)(H,20,22)/t13?,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM381823
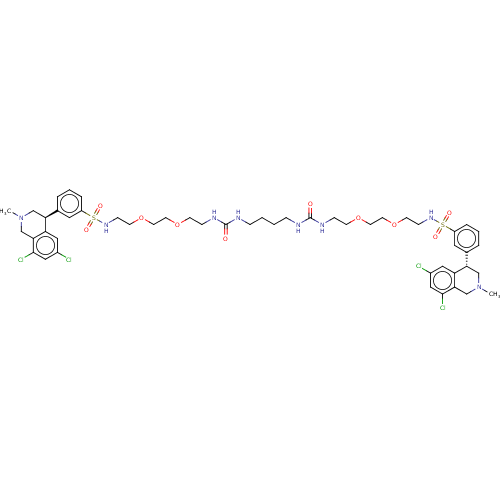
(CVD-0019453 | US10272079, Compound 002 | US1027207...)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)NCCCCNC(=O)NCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| Show InChI InChI=1S/C50H66Cl4N8O10S2/c1-61-31-43(41-27-37(51)29-47(53)45(41)33-61)35-7-5-9-39(25-35)73(65,66)59-15-19-71-23-21-69-17-13-57-49(63)55-11-3-4-12-56-50(64)58-14-18-70-22-24-72-20-16-60-74(67,68)40-10-6-8-36(26-40)44-32-62(2)34-46-42(44)28-38(52)30-48(46)54/h5-10,25-30,43-44,59-60H,3-4,11-24,31-34H2,1-2H3,(H2,55,57,63)(H2,56,58,64)/t43-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM50601173
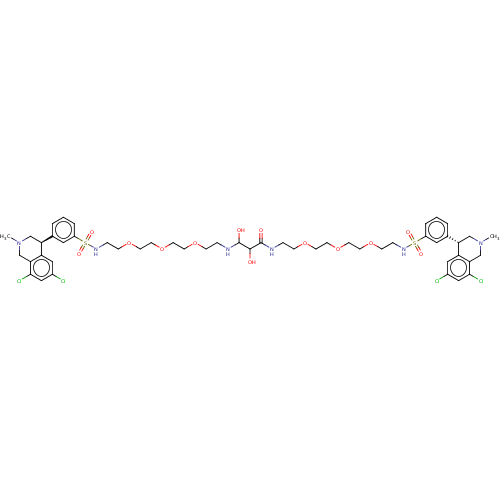
(CHEMBL5176968)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCOCCNC(O)C(O)C(=O)NCCOCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070227
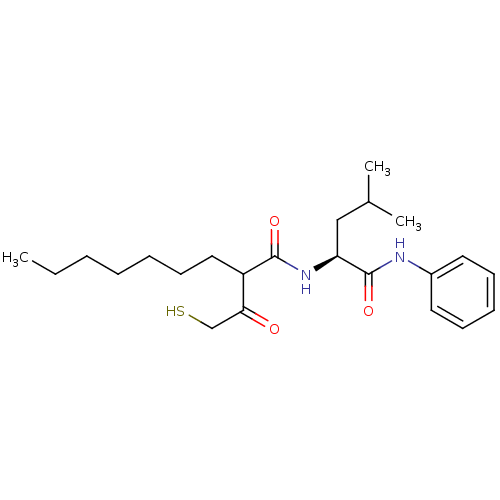
(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-3-methyl-...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C23H36N2O3S/c1-4-5-6-7-11-14-19(21(26)16-29)22(27)25-20(15-17(2)3)23(28)24-18-12-9-8-10-13-18/h8-10,12-13,17,19-20,29H,4-7,11,14-16H2,1-3H3,(H,24,28)(H,25,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070247

(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-2-cyclohe...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C28H44N2O3S/c1-2-3-4-5-12-17-24(26(31)21-34)27(32)30-25(20-23-15-10-7-11-16-23)28(33)29-19-18-22-13-8-6-9-14-22/h6,8-9,13-14,23-25,34H,2-5,7,10-12,15-21H2,1H3,(H,29,33)(H,30,32)/t24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM381826
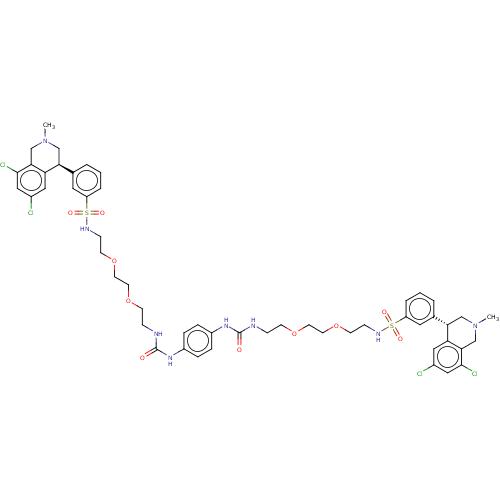
(US10272079, Compound 003)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)Nc2ccc(NC(=O)NCCOCCOCCNS(=O)(=O)c3cccc(c3)[C@@H]3CN(C)Cc4c(Cl)cc(Cl)cc34)cc2)c2cc(Cl)cc(Cl)c2C1 |r| Show InChI InChI=1S/C52H62Cl4N8O10S2/c1-63-31-45(43-27-37(53)29-49(55)47(43)33-63)35-5-3-7-41(25-35)75(67,68)59-15-19-73-23-21-71-17-13-57-51(65)61-39-9-11-40(12-10-39)62-52(66)58-14-18-72-22-24-74-20-16-60-76(69,70)42-8-4-6-36(26-42)46-32-64(2)34-48-44(46)28-38(54)30-50(48)56/h3-12,25-30,45-46,59-60H,13-24,31-34H2,1-2H3,(H2,57,61,65)(H2,58,62,66)/t45-,46-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM50601180

(CHEMBL5170002)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)CCC(=O)NCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM50601178
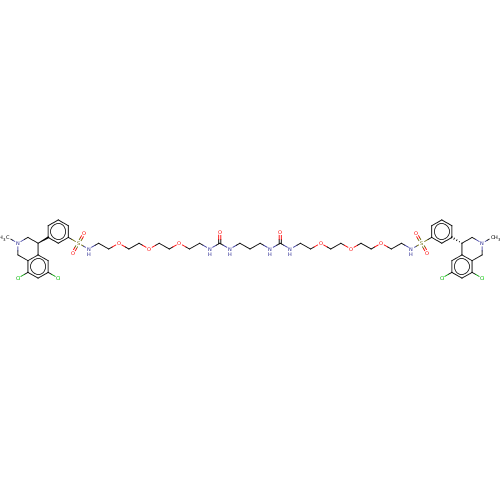
(CHEMBL5202545)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCOCCNC(=O)NCCCNC(=O)NCCOCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50070250

(2-(1-Hydroxy-2-mercapto-ethyl)-5-phenyl-pentanoic ...)Show SMILES OC(CS)C(CCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H42N2O3S/c33-28(22-36)26(18-10-17-23-11-4-1-5-12-23)29(34)32-27(21-25-15-8-3-9-16-25)30(35)31-20-19-24-13-6-2-7-14-24/h1-2,4-7,11-14,25-28,33,36H,3,8-10,15-22H2,(H,31,35)(H,32,34)/t26?,27-,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against stromelysin-3 (MMP-3) |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM381826

(US10272079, Compound 003)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)Nc2ccc(NC(=O)NCCOCCOCCNS(=O)(=O)c3cccc(c3)[C@@H]3CN(C)Cc4c(Cl)cc(Cl)cc34)cc2)c2cc(Cl)cc(Cl)c2C1 |r| Show InChI InChI=1S/C52H62Cl4N8O10S2/c1-63-31-45(43-27-37(53)29-49(55)47(43)33-63)35-5-3-7-41(25-35)75(67,68)59-15-19-73-23-21-71-17-13-57-51(65)61-39-9-11-40(12-10-39)62-52(66)58-14-18-72-22-24-74-20-16-60-76(69,70)42-8-4-6-36(26-42)46-32-64(2)34-48-44(46)28-38(54)30-50(48)56/h3-12,25-30,45-46,59-60H,13-24,31-34H2,1-2H3,(H2,57,61,65)(H2,58,62,66)/t45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335797
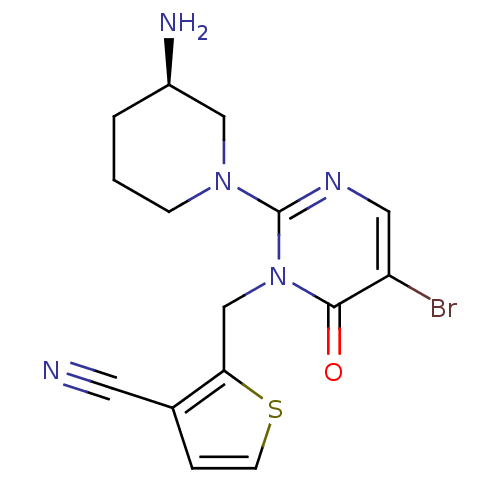
(2-[2-(3-(R)-Aminopyrimidin-1-yl)-5-bromo-6-oxo-6H-...)Show SMILES N[C@@H]1CCCN(C1)c1ncc(Br)c(=O)n1Cc1sccc1C#N |r| Show InChI InChI=1S/C15H16BrN5OS/c16-12-7-19-15(20-4-1-2-11(18)8-20)21(14(12)22)9-13-10(6-17)3-5-23-13/h3,5,7,11H,1-2,4,8-9,18H2/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM426615
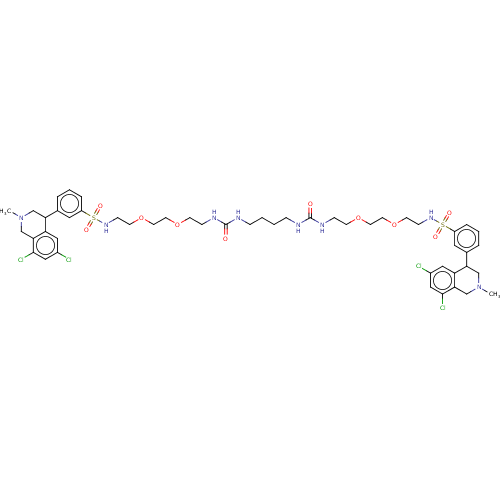
(N,N′-(10,17-dioxo-3,6,21,24-tetraoxa-9,11,16...)Show SMILES CN1CC(c2cccc(c2)S(=O)(=O)NCCOCCOCCNC(=O)NCCCCNC(=O)NCCOCCOCCNS(=O)(=O)c2cccc(c2)C2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 Show InChI InChI=1S/C50H66Cl4N8O10S2/c1-61-31-43(41-27-37(51)29-47(53)45(41)33-61)35-7-5-9-39(25-35)73(65,66)59-15-19-71-23-21-69-17-13-57-49(63)55-11-3-4-12-56-50(64)58-14-18-70-22-24-72-20-16-60-74(67,68)40-10-6-8-36(26-40)44-32-62(2)34-46-42(44)28-38(52)30-48(46)54/h5-10,25-30,43-44,59-60H,3-4,11-24,31-34H2,1-2H3,(H2,55,57,63)(H2,56,58,64) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM50601172

(CHEMBL5183104)Show SMILES CN1CC(c2ccc(cc2)S(=O)(=O)NCCOCCOCCNC(O)C(O)C(=O)NCCOCCOCCNS(=O)(=O)c2ccc(cc2)C2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335768
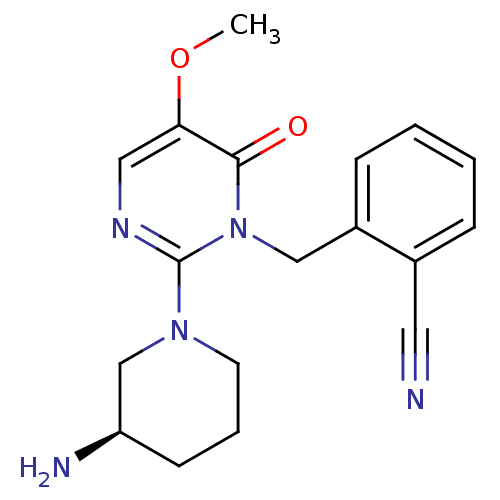
(2-[2-(3-(R)-Aminopiperidin-1-yl)-5-methoxy-6-oxo-6...)Show SMILES COc1cnc(N2CCC[C@@H](N)C2)n(Cc2ccccc2C#N)c1=O |r| Show InChI InChI=1S/C18H21N5O2/c1-25-16-10-21-18(22-8-4-7-15(20)12-22)23(17(16)24)11-14-6-3-2-5-13(14)9-19/h2-3,5-6,10,15H,4,7-8,11-12,20H2,1H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335784
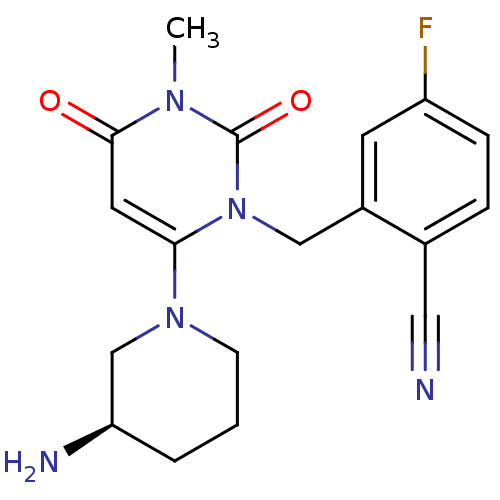
(2-[6-(3-Aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,...)Show SMILES Cn1c(=O)cc(N2CCC[C@@H](N)C2)n(Cc2cc(F)ccc2C#N)c1=O |r| Show InChI InChI=1S/C18H20FN5O2/c1-22-17(25)8-16(23-6-2-3-15(21)11-23)24(18(22)26)10-13-7-14(19)5-4-12(13)9-20/h4-5,7-8,15H,2-3,6,10-11,21H2,1H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070226
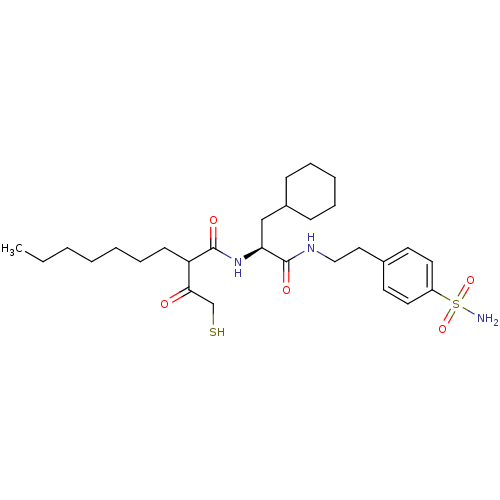
(2-(2-Mercapto-acetyl)-nonanoic acid {(S)-2-cyclohe...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C28H45N3O5S2/c1-2-3-4-5-9-12-24(26(32)20-37)27(33)31-25(19-22-10-7-6-8-11-22)28(34)30-18-17-21-13-15-23(16-14-21)38(29,35)36/h13-16,22,24-25,37H,2-12,17-20H2,1H3,(H,30,34)(H,31,33)(H2,29,35,36)/t24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM16274
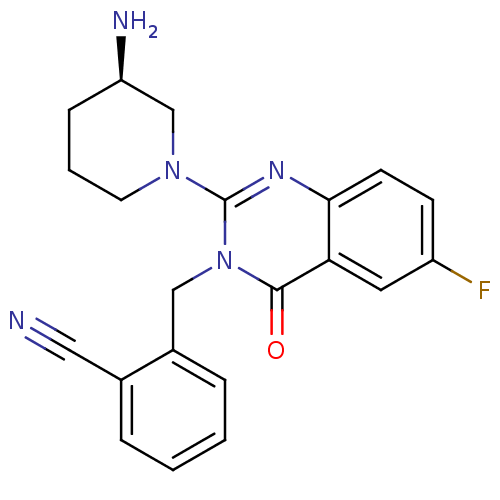
(2-({2-[(3R)-3-aminopiperidin-1-yl]-6-fluoro-4-oxo-...)Show SMILES N[C@@H]1CCCN(C1)c1nc2ccc(F)cc2c(=O)n1Cc1ccccc1C#N |r| Show InChI InChI=1S/C21H20FN5O/c22-16-7-8-19-18(10-16)20(28)27(12-15-5-2-1-4-14(15)11-23)21(25-19)26-9-3-6-17(24)13-26/h1-2,4-5,7-8,10,17H,3,6,9,12-13,24H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Takeda Pharmaceutical Company Ltd.
| Assay Description
Compounds were tested for their ability to inhibit DPP enzymes mediated cleavage of Ala-Pro-7-amido-4-trifluoromethylcoumarin in a fluorogenic assay.... |
J Med Chem 50: 2297-300 (2007)
Article DOI: 10.1021/jm070104l
BindingDB Entry DOI: 10.7270/Q2TM78C5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM16281

(2-({2-[(3R)-3-aminopiperidin-1-yl]-7-fluoro-6-meth...)Show SMILES COc1cc2c(cc1F)nc(N1CCC[C@@H](N)C1)n(Cc1ccccc1C#N)c2=O |r| Show InChI InChI=1S/C22H22FN5O2/c1-30-20-9-17-19(10-18(20)23)26-22(27-8-4-7-16(25)13-27)28(21(17)29)12-15-6-3-2-5-14(15)11-24/h2-3,5-6,9-10,16H,4,7-8,12-13,25H2,1H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Takeda Pharmaceutical Company Ltd.
| Assay Description
Compounds were tested for their ability to inhibit DPP enzymes mediated cleavage of Ala-Pro-7-amido-4-trifluoromethylcoumarin in a fluorogenic assay.... |
J Med Chem 50: 2297-300 (2007)
Article DOI: 10.1021/jm070104l
BindingDB Entry DOI: 10.7270/Q2TM78C5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335792
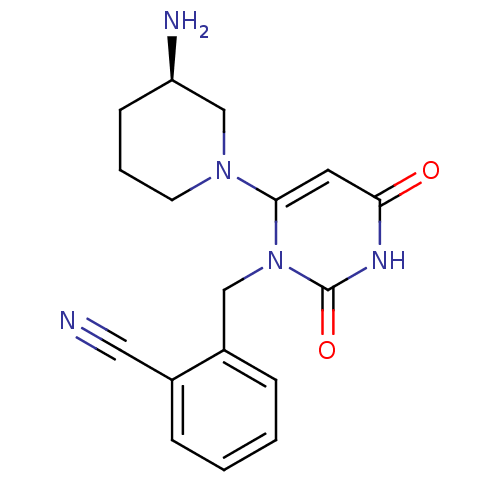
(2-{6-[3(R)-Aminopiperidin-1-yl]-2,4-dioxo-3,4-dihy...)Show SMILES N[C@@H]1CCCN(C1)c1cc(=O)[nH]c(=O)n1Cc1ccccc1C#N |r| Show InChI InChI=1S/C17H19N5O2/c18-9-12-4-1-2-5-13(12)10-22-16(8-15(23)20-17(22)24)21-7-3-6-14(19)11-21/h1-2,4-5,8,14H,3,6-7,10-11,19H2,(H,20,23,24)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317946
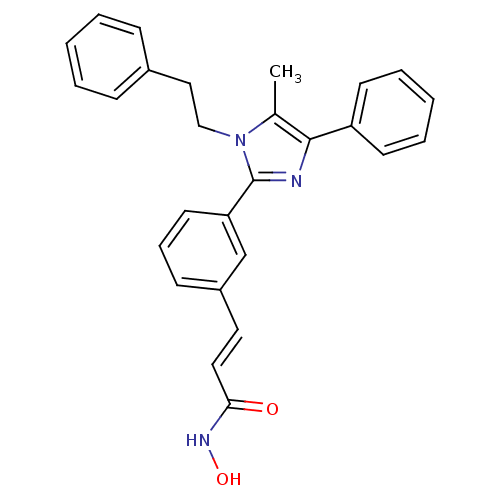
(CHEMBL1095451 | N-hydroxy-3-(3-(5-methyl-1-pheneth...)Show SMILES Cc1c(nc(-c2cccc(\C=C\C(=O)NO)c2)n1CCc1ccccc1)-c1ccccc1 Show InChI InChI=1S/C27H25N3O2/c1-20-26(23-12-6-3-7-13-23)28-27(30(20)18-17-21-9-4-2-5-10-21)24-14-8-11-22(19-24)15-16-25(31)29-32/h2-16,19,32H,17-18H2,1H3,(H,29,31)/b16-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317947
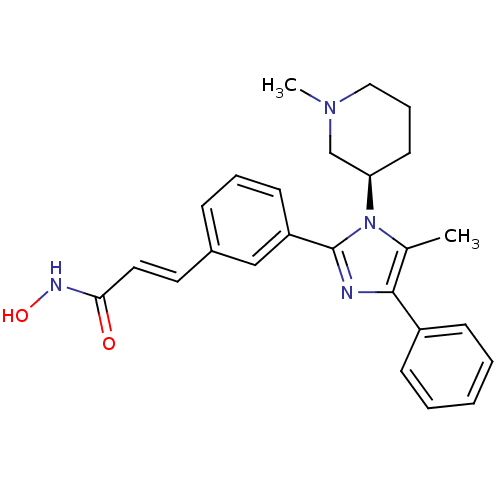
((R)-N-hydroxy-3-(3-(5-methyl-1-(1-methylpiperidin-...)Show SMILES CN1CCC[C@H](C1)n1c(C)c(nc1-c1cccc(\C=C\C(=O)NO)c1)-c1ccccc1 |r| Show InChI InChI=1S/C25H28N4O2/c1-18-24(20-9-4-3-5-10-20)26-25(29(18)22-12-7-15-28(2)17-22)21-11-6-8-19(16-21)13-14-23(30)27-31/h3-6,8-11,13-14,16,22,31H,7,12,15,17H2,1-2H3,(H,27,30)/b14-13+/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317949
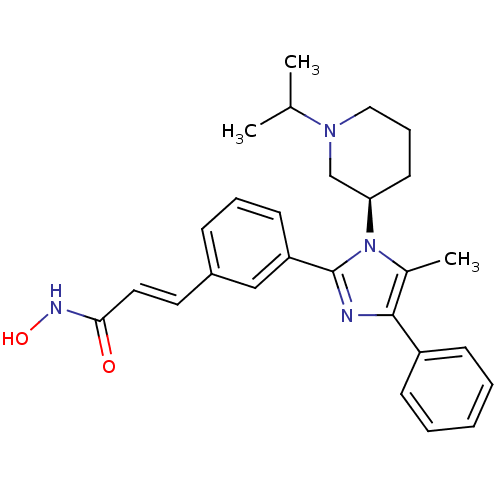
((R)-N-hydroxy-3-(3-(1-(1-isopropylpiperidin-3-yl)-...)Show SMILES CC(C)N1CCC[C@H](C1)n1c(C)c(nc1-c1cccc(\C=C\C(=O)NO)c1)-c1ccccc1 |r| Show InChI InChI=1S/C27H32N4O2/c1-19(2)30-16-8-13-24(18-30)31-20(3)26(22-10-5-4-6-11-22)28-27(31)23-12-7-9-21(17-23)14-15-25(32)29-33/h4-7,9-12,14-15,17,19,24,33H,8,13,16,18H2,1-3H3,(H,29,32)/b15-14+/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317960

(3-(3-(5-benzyl-4-methyl-1-phenethyl-1H-imidazol-2-...)Show SMILES Cc1nc(-c2cccc(\C=C\C(=O)NO)c2)n(CCc2ccccc2)c1Cc1ccccc1 Show InChI InChI=1S/C28H27N3O2/c1-21-26(20-23-11-6-3-7-12-23)31(18-17-22-9-4-2-5-10-22)28(29-21)25-14-8-13-24(19-25)15-16-27(32)30-33/h2-16,19,33H,17-18,20H2,1H3,(H,30,32)/b16-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063920
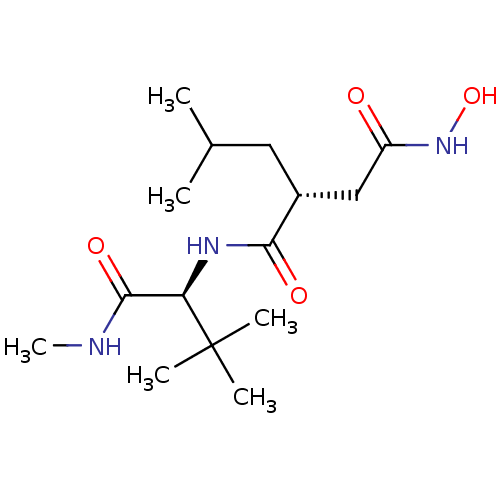
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-10(8-11(19)18-22)13(20)17-12(14(21)16-6)15(3,4)5/h9-10,12,22H,7-8H2,1-6H3,(H,16,21)(H,17,20)(H,18,19)/t10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1, Collagenase-1 |
Bioorg Med Chem Lett 8: 1163-8 (1999)
BindingDB Entry DOI: 10.7270/Q2348JH9 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50070256

((R)-2-((S)-2-Hydroxy-3-mercapto-propyl)-nonanoic a...)Show SMILES CCCCCCC[C@H](C[C@H](O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C19H38N2O3S/c1-6-7-8-9-10-11-14(12-15(22)13-25)17(23)21-16(18(24)20-5)19(2,3)4/h14-16,22,25H,6-13H2,1-5H3,(H,20,24)(H,21,23)/t14-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1, Collagenase-1 |
Bioorg Med Chem Lett 8: 1163-8 (1999)
BindingDB Entry DOI: 10.7270/Q2348JH9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070223
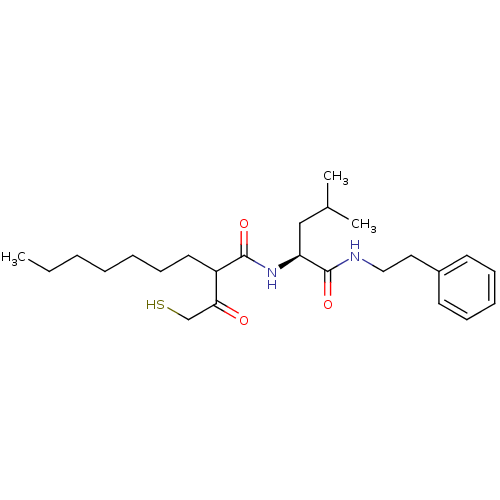
(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-3-methyl-...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C25H40N2O3S/c1-4-5-6-7-11-14-21(23(28)18-31)24(29)27-22(17-19(2)3)25(30)26-16-15-20-12-9-8-10-13-20/h8-10,12-13,19,21-22,31H,4-7,11,14-18H2,1-3H3,(H,26,30)(H,27,29)/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335757

(2-[2-(3-(R)-Aminopiperidin-1-yl)-5-ethynyl-6-oxo-6...)Show SMILES N[C@@H]1CCCN(C1)c1ncc(C#C)c(=O)n1Cc1ccccc1C#N |r| Show InChI InChI=1S/C19H19N5O/c1-2-14-11-22-19(23-9-5-8-17(21)13-23)24(18(14)25)12-16-7-4-3-6-15(16)10-20/h1,3-4,6-7,11,17H,5,8-9,12-13,21H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335759

(2-[2-(3-(R)-Aminopiperidin-1-yl)-6-oxo-5-(1H-pyrro...)Show SMILES N[C@@H]1CCCN(C1)c1ncc(-c2cc[nH]c2)c(=O)n1Cc1ccccc1C#N |r| Show InChI InChI=1S/C21H22N6O/c22-10-15-4-1-2-5-17(15)13-27-20(28)19(16-7-8-24-11-16)12-25-21(27)26-9-3-6-18(23)14-26/h1-2,4-5,7-8,11-12,18,24H,3,6,9,13-14,23H2/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335774

(2-((2-(3-(R)-Aminopiperidin-1-yl)-5-bromo-6-oxopyr...)Show SMILES N[C@@H]1CCCN(C1)c1ncc(Br)c(=O)n1Cc1ccccc1C#N |r| Show InChI InChI=1S/C17H18BrN5O/c18-15-9-21-17(22-7-3-6-14(20)11-22)23(16(15)24)10-13-5-2-1-4-12(13)8-19/h1-2,4-5,9,14H,3,6-7,10-11,20H2/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM16273
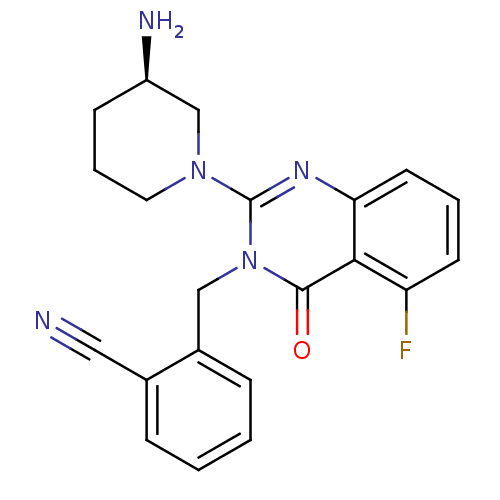
(2-({2-[(3R)-3-aminopiperidin-1-yl]-5-fluoro-4-oxo-...)Show SMILES N[C@@H]1CCCN(C1)c1nc2cccc(F)c2c(=O)n1Cc1ccccc1C#N |r| Show InChI InChI=1S/C21H20FN5O/c22-17-8-3-9-18-19(17)20(28)27(12-15-6-2-1-5-14(15)11-23)21(25-18)26-10-4-7-16(24)13-26/h1-3,5-6,8-9,16H,4,7,10,12-13,24H2/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Takeda Pharmaceutical Company Ltd.
| Assay Description
Compounds were tested for their ability to inhibit DPP enzymes mediated cleavage of Ala-Pro-7-amido-4-trifluoromethylcoumarin in a fluorogenic assay.... |
J Med Chem 50: 2297-300 (2007)
Article DOI: 10.1021/jm070104l
BindingDB Entry DOI: 10.7270/Q2TM78C5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM16275
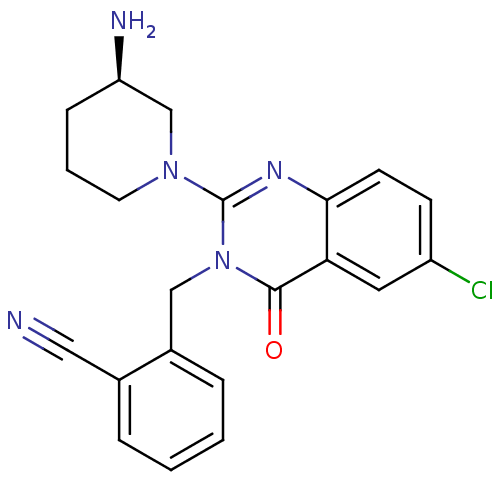
(2-({2-[(3R)-3-aminopiperidin-1-yl]-6-chloro-4-oxo-...)Show SMILES N[C@@H]1CCCN(C1)c1nc2ccc(Cl)cc2c(=O)n1Cc1ccccc1C#N |r| Show InChI InChI=1S/C21H20ClN5O/c22-16-7-8-19-18(10-16)20(28)27(12-15-5-2-1-4-14(15)11-23)21(25-19)26-9-3-6-17(24)13-26/h1-2,4-5,7-8,10,17H,3,6,9,12-13,24H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Takeda Pharmaceutical Company Ltd.
| Assay Description
Compounds were tested for their ability to inhibit DPP enzymes mediated cleavage of Ala-Pro-7-amido-4-trifluoromethylcoumarin in a fluorogenic assay.... |
J Med Chem 50: 2297-300 (2007)
Article DOI: 10.1021/jm070104l
BindingDB Entry DOI: 10.7270/Q2TM78C5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM16279
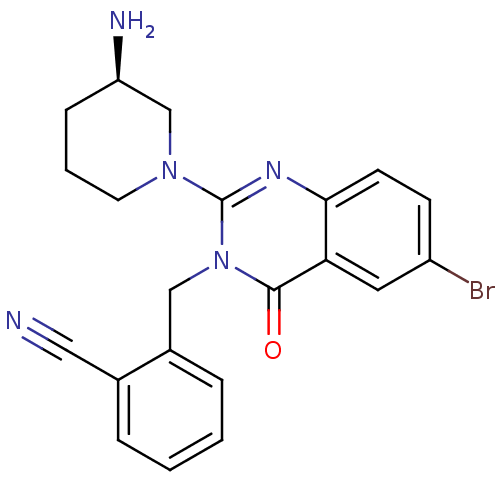
(2-({2-[(3R)-3-aminopiperidin-1-yl]-6-bromo-4-oxo-3...)Show SMILES N[C@@H]1CCCN(C1)c1nc2ccc(Br)cc2c(=O)n1Cc1ccccc1C#N |r| Show InChI InChI=1S/C21H20BrN5O/c22-16-7-8-19-18(10-16)20(28)27(12-15-5-2-1-4-14(15)11-23)21(25-19)26-9-3-6-17(24)13-26/h1-2,4-5,7-8,10,17H,3,6,9,12-13,24H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Takeda Pharmaceutical Company Ltd.
| Assay Description
Compounds were tested for their ability to inhibit DPP enzymes mediated cleavage of Ala-Pro-7-amido-4-trifluoromethylcoumarin in a fluorogenic assay.... |
J Med Chem 50: 2297-300 (2007)
Article DOI: 10.1021/jm070104l
BindingDB Entry DOI: 10.7270/Q2TM78C5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335756
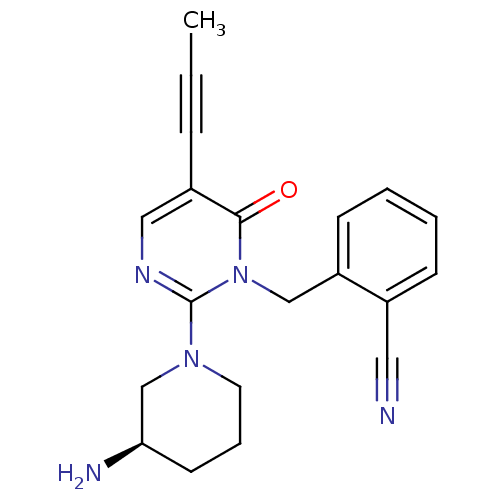
(2-[2-(3-(R)-Aminopiperidin-1-yl)-5-prop-1-ynyl-6-o...)Show SMILES CC#Cc1cnc(N2CCC[C@@H](N)C2)n(Cc2ccccc2C#N)c1=O |r| Show InChI InChI=1S/C20H21N5O/c1-2-6-16-12-23-20(24-10-5-9-18(22)14-24)25(19(16)26)13-17-8-4-3-7-15(17)11-21/h3-4,7-8,12,18H,5,9-10,13-14,22H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335771

(2-[2-(3-(R)-Aminopiperidin-1-yl)-5-iodo-6-oxo-6H-p...)Show SMILES N[C@@H]1CCCN(C1)c1ncc(I)c(=O)n1Cc1ccccc1C#N |r| Show InChI InChI=1S/C17H18IN5O/c18-15-9-21-17(22-7-3-6-14(20)11-22)23(16(15)24)10-13-5-2-1-4-12(13)8-19/h1-2,4-5,9,14H,3,6-7,10-11,20H2/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070229

(2-(2-Mercapto-acetyl)-4-methyl-pentanoic acid ((S)...)Show SMILES CNC(=O)[C@@H](NC(=O)C(CC(C)C)C(=O)CS)C(C)(C)C Show InChI InChI=1S/C15H28N2O3S/c1-9(2)7-10(11(18)8-21)13(19)17-12(14(20)16-6)15(3,4)5/h9-10,12,21H,7-8H2,1-6H3,(H,16,20)(H,17,19)/t10?,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335798

(2-[2-(3-(R)-Aminopiperidin-1-yl)-6-oxo-5-((E)-2-py...)Show SMILES N[C@@H]1CCCN(C1)c1ncc(\C=C\c2cccnc2)c(=O)n1Cc1ccccc1C#N |r| Show InChI InChI=1S/C24H24N6O/c25-13-19-6-1-2-7-21(19)16-30-23(31)20(10-9-18-5-3-11-27-14-18)15-28-24(30)29-12-4-8-22(26)17-29/h1-3,5-7,9-11,14-15,22H,4,8,12,16-17,26H2/b10-9+/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335793
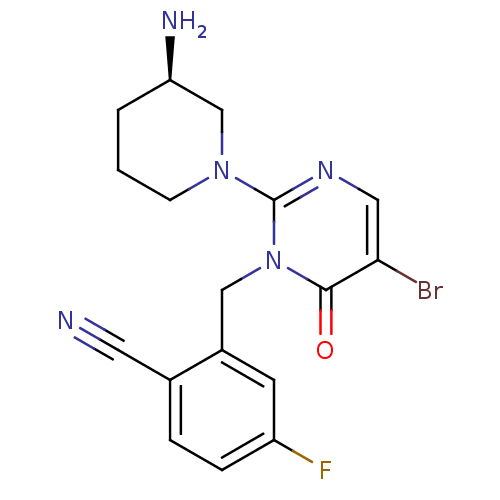
(2-((2-(3-(R)-Aminopiperidin-1-yl)-5-bromo-6-oxopyr...)Show SMILES N[C@@H]1CCCN(C1)c1ncc(Br)c(=O)n1Cc1cc(F)ccc1C#N |r| Show InChI InChI=1S/C17H17BrFN5O/c18-15-8-22-17(23-5-1-2-14(21)10-23)24(16(15)25)9-12-6-13(19)4-3-11(12)7-20/h3-4,6,8,14H,1-2,5,9-10,21H2/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070239
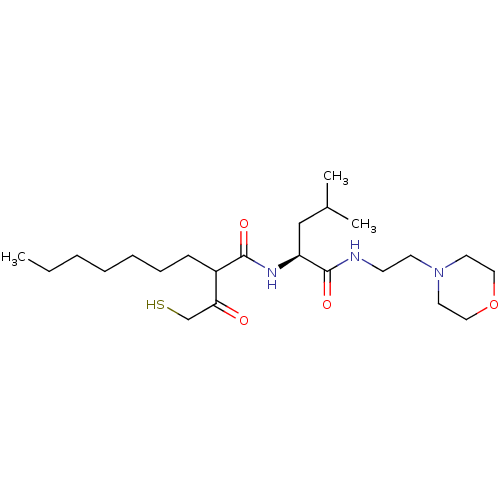
(2-(2-Mercapto-acetyl)-nonanoic acid [(S)-3-methyl-...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C23H43N3O4S/c1-4-5-6-7-8-9-19(21(27)17-31)22(28)25-20(16-18(2)3)23(29)24-10-11-26-12-14-30-15-13-26/h18-20,31H,4-17H2,1-3H3,(H,24,29)(H,25,28)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335777

(2-[2-(3-(R)-Aminopiperidin-1-yl)-5-fluoro-6-oxo-6H...)Show SMILES N[C@@H]1CCCN(C1)c1ncc(F)c(=O)n1Cc1ccccc1C#N |r| Show InChI InChI=1S/C17H18FN5O/c18-15-9-21-17(22-7-3-6-14(20)11-22)23(16(15)24)10-13-5-2-1-4-12(13)8-19/h1-2,4-5,9,14H,3,6-7,10-11,20H2/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317957
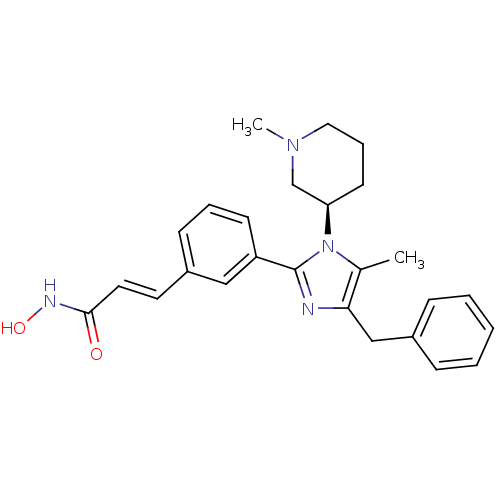
((R)-3-(3-(4-benzyl-5-methyl-1-(1-methylpiperidin-3...)Show SMILES CN1CCC[C@H](C1)n1c(C)c(Cc2ccccc2)nc1-c1cccc(\C=C\C(=O)NO)c1 |r| Show InChI InChI=1S/C26H30N4O2/c1-19-24(17-20-8-4-3-5-9-20)27-26(30(19)23-12-7-15-29(2)18-23)22-11-6-10-21(16-22)13-14-25(31)28-32/h3-6,8-11,13-14,16,23,32H,7,12,15,17-18H2,1-2H3,(H,28,31)/b14-13+/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317954
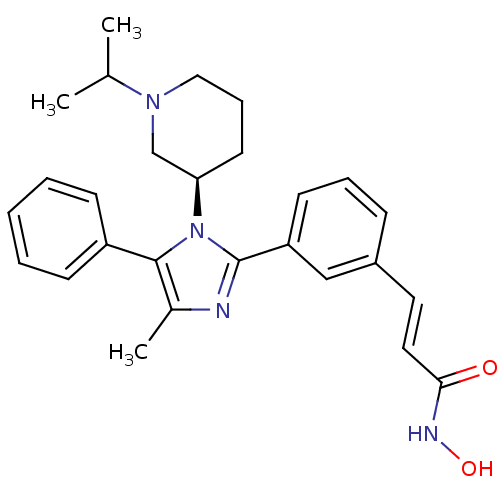
((R)-N-hydroxy-3-(3-(1-(1-isopropylpiperidin-3-yl)-...)Show SMILES CC(C)N1CCC[C@H](C1)n1c(nc(C)c1-c1ccccc1)-c1cccc(\C=C\C(=O)NO)c1 |r| Show InChI InChI=1S/C27H32N4O2/c1-19(2)30-16-8-13-24(18-30)31-26(22-10-5-4-6-11-22)20(3)28-27(31)23-12-7-9-21(17-23)14-15-25(32)29-33/h4-7,9-12,14-15,17,19,24,33H,8,13,16,18H2,1-3H3,(H,29,32)/b15-14+/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317948
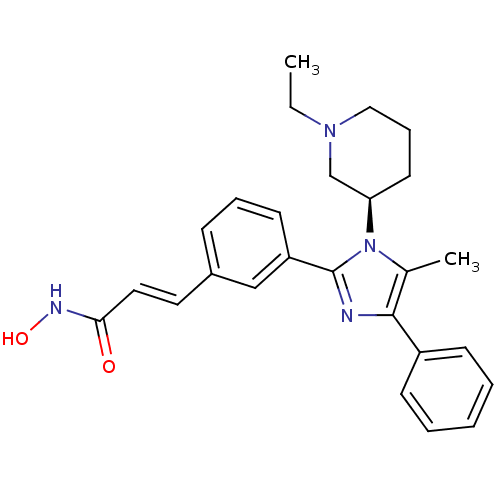
(6-(1-((R)-1-ethylpiperidin-3-yl)-5-methyl-4-phenyl...)Show SMILES CCN1CCC[C@H](C1)n1c(C)c(nc1-c1cccc(\C=C\C(=O)NO)c1)-c1ccccc1 |r| Show InChI InChI=1S/C26H30N4O2/c1-3-29-16-8-13-23(18-29)30-19(2)25(21-10-5-4-6-11-21)27-26(30)22-12-7-9-20(17-22)14-15-24(31)28-32/h4-7,9-12,14-15,17,23,32H,3,8,13,16,18H2,1-2H3,(H,28,31)/b15-14+/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Sodium/hydrogen exchanger 3
(Rattus norvegicus) | BDBM50601178

(CHEMBL5202545)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCOCCNC(=O)NCCCNC(=O)NCCOCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50335775

(2-[2-(3-(R)-Aminopiperidin-1-yl)-5-chloro-6-oxo-6H...)Show SMILES N[C@@H]1CCCN(C1)c1ncc(Cl)c(=O)n1Cc1ccccc1C#N |r| Show InChI InChI=1S/C17H18ClN5O/c18-15-9-21-17(22-7-3-6-14(20)11-22)23(16(15)24)10-13-5-2-1-4-12(13)8-19/h1-2,4-5,9,14H,3,6-7,10-11,20H2/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM16285
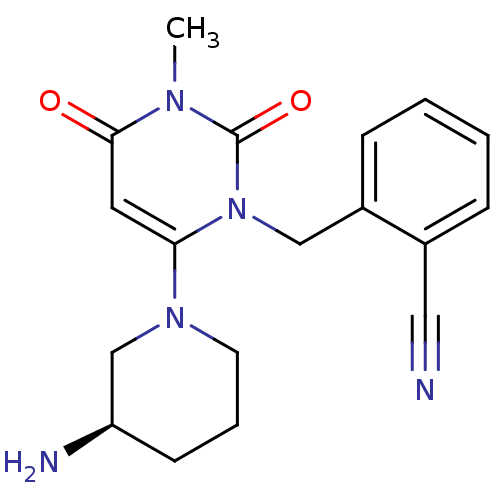
(2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-di...)Show SMILES Cn1c(=O)cc(N2CCC[C@@H](N)C2)n(Cc2ccccc2C#N)c1=O |r| Show InChI InChI=1S/C18H21N5O2/c1-21-17(24)9-16(22-8-4-7-15(20)12-22)23(18(21)25)11-14-6-3-2-5-13(14)10-19/h2-3,5-6,9,15H,4,7-8,11-12,20H2,1H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
J Med Chem 54: 510-24 (2011)
Article DOI: 10.1021/jm101016w
BindingDB Entry DOI: 10.7270/Q24X5828 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/hydrogen exchanger 3
(Homo sapiens (Human)) | BDBM50601181
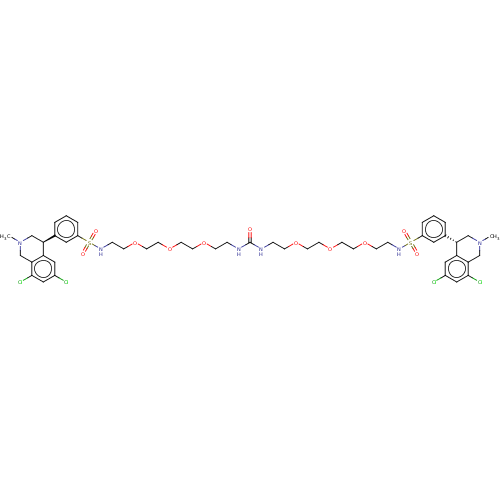
(CHEMBL5170188)Show SMILES CN1C[C@@H](c2cccc(c2)S(=O)(=O)NCCOCCOCCOCCNC(=O)NCCOCCOCCOCCNS(=O)(=O)c2cccc(c2)[C@@H]2CN(C)Cc3c(Cl)cc(Cl)cc23)c2cc(Cl)cc(Cl)c2C1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00037
BindingDB Entry DOI: 10.7270/Q2DB85X0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317965

(CHEMBL1099096 | N-hydroxy-3-(3-(1-phenethyl-1H-ben...)Show SMILES ONC(=O)\C=C\c1cccc(c1)-c1nc2ccccc2n1CCc1ccccc1 Show InChI InChI=1S/C24H21N3O2/c28-23(26-29)14-13-19-9-6-10-20(17-19)24-25-21-11-4-5-12-22(21)27(24)16-15-18-7-2-1-3-8-18/h1-14,17,29H,15-16H2,(H,26,28)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317953

((R)-3-(3-(1-(1-ethylpiperidin-3-yl)-4-methyl-5-phe...)Show SMILES CCN1CCC[C@H](C1)n1c(nc(C)c1-c1ccccc1)-c1cccc(\C=C\C(=O)NO)c1 |r| Show InChI InChI=1S/C26H30N4O2/c1-3-29-16-8-13-23(18-29)30-25(21-10-5-4-6-11-21)19(2)27-26(30)22-12-7-9-20(17-22)14-15-24(31)28-32/h4-7,9-12,14-15,17,23,32H,3,8,13,16,18H2,1-2H3,(H,28,31)/b15-14+/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Bioorg Med Chem Lett 20: 3138-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.092
BindingDB Entry DOI: 10.7270/Q2514058 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data