

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309873 (4-(6-Chlorooxazolo[4,5-b]pyridin-2-yl)-1,4-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401002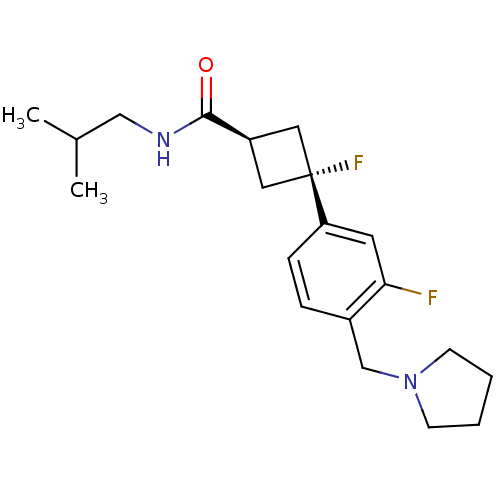 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50309864 (4-(5-Chlorobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401007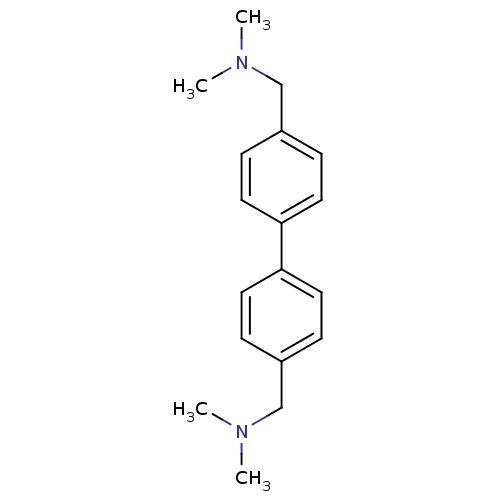 (CHEMBL209478) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401007 (CHEMBL209478) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401004 (CHEMBL2206291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401004 (CHEMBL2206291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401003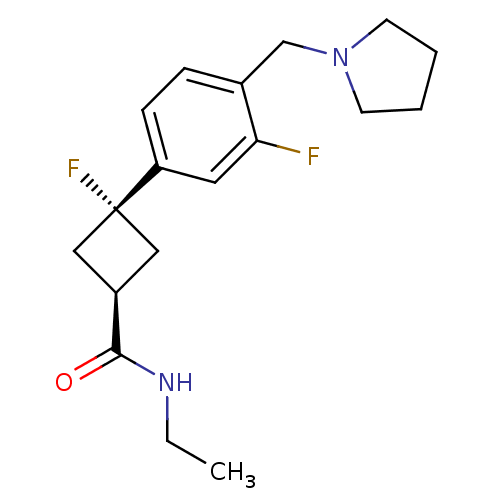 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401003 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309897 (2-(1,4-Diazabicyclo[3.2.2]nonan-4-yl)-5-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309864 (4-(5-Chlorobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309872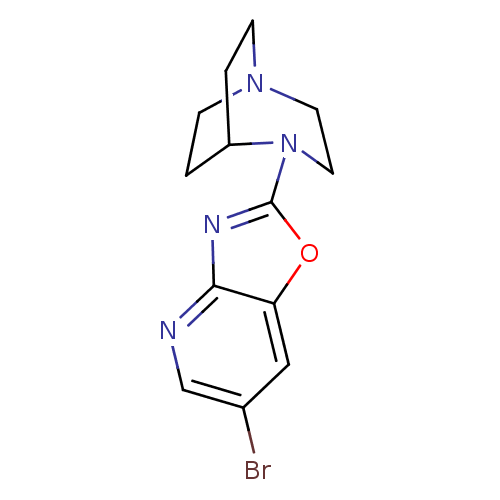 (4-(6-Bromooxazolo[4,5-b]pyridin-2-yl)-1,4-diazabic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309865 (4-(5-Bromobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401003 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401003 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309880 (4-(6-Phenyloxazolo[5,4-b]pyridin-2-yl)-1,4-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309879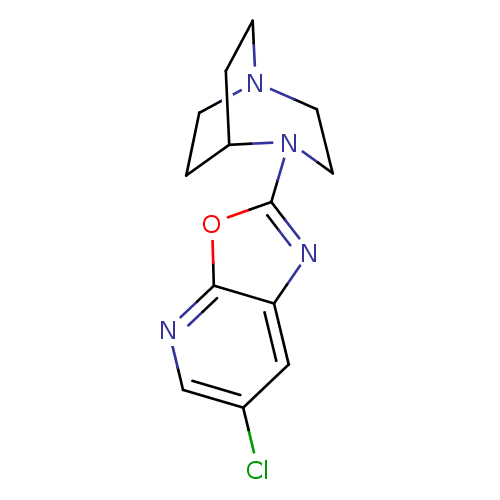 (2-(6-Chlorooxazolo[5,4-b]pyridin-2-yl)-2,5-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309888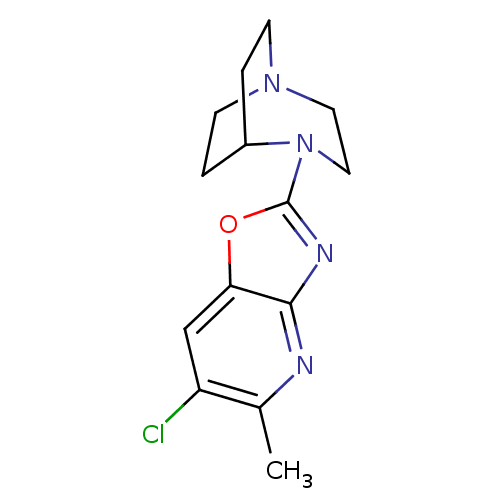 (4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309892 (2-(5-Phenyloxazolo[4,5-b]pyridin-2-yl)-2,5-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401005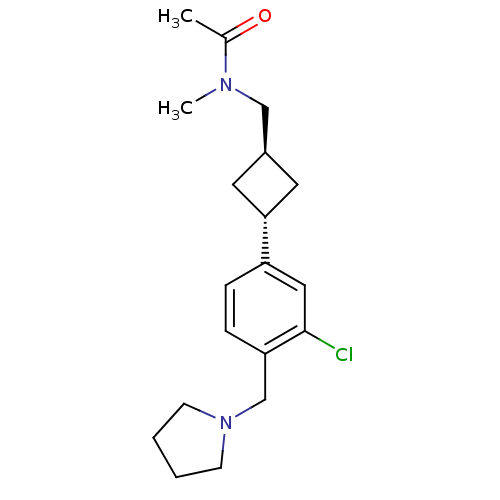 (CHEMBL2206290) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401005 (CHEMBL2206290) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50309866 (4-(5-Methylbenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309893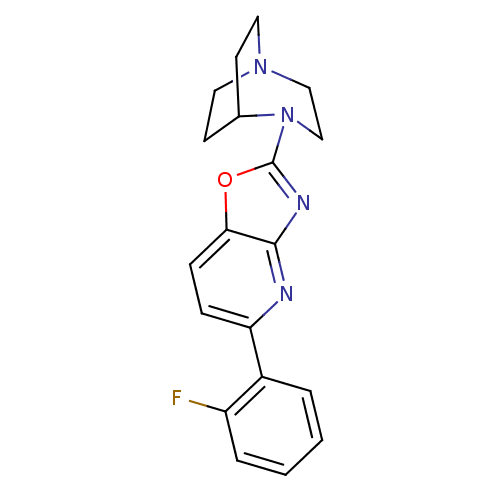 (2-(5-(2-Fluorophenyl)oxazolo[4,5-b]pyridin-2-yl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated 10... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated 10... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50309863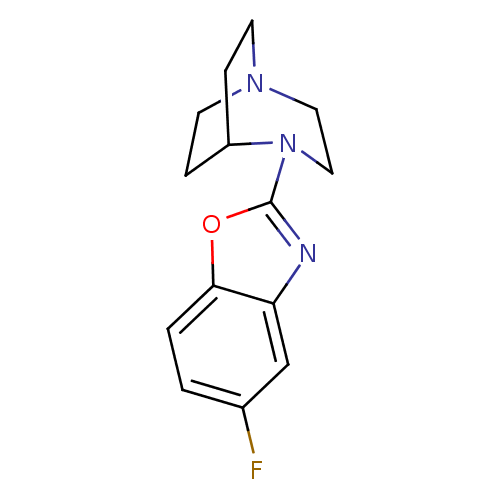 (4-(5-Fluorobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401004 (CHEMBL2206291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401004 (CHEMBL2206291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309886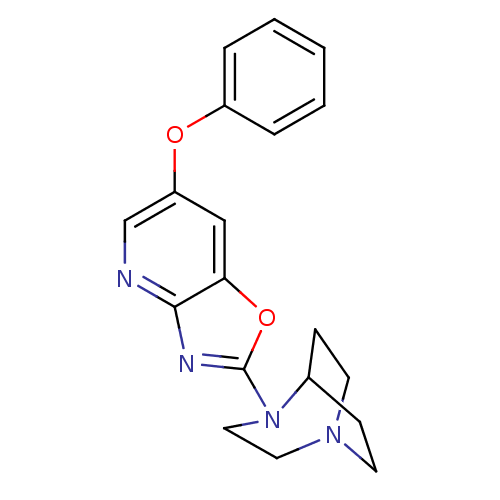 (2-(6-Phenoxyoxazolo[4,5-b]pyridin-2-yl)-2,5-diazab...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309891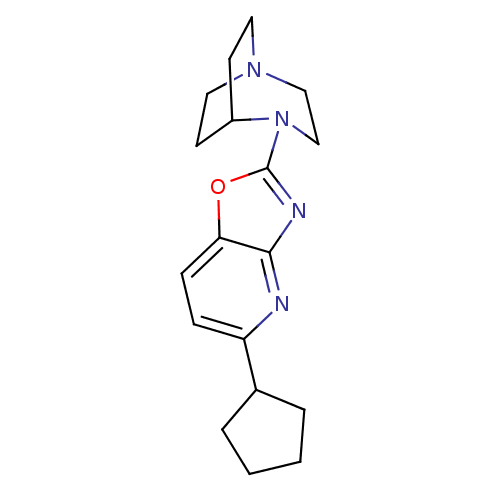 (2-(5-Cyclopentyloxazolo[4,5-b]pyridin-2-yl)-2,5-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309882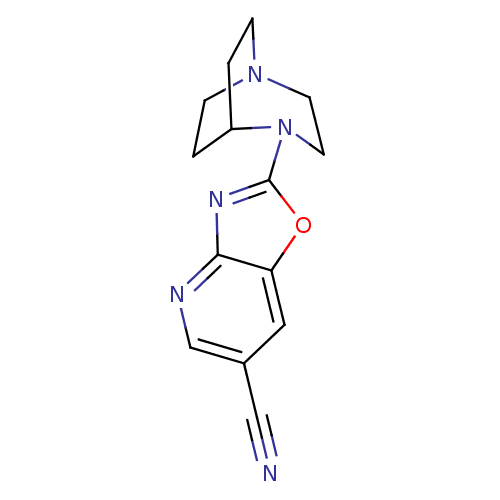 (4-(6-Cyanooxazolo[4,5-b]pyridin-2-yl)-1,4-diazabic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309875 (4-(6-Methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309878 (4-(6-Phenyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from rat histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401001 (CHEMBL2206288) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from rat histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated 10... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401002 (CHEMBL2206292) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated 10... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401006 (CHEMBL2206289) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50401006 (CHEMBL2206289) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401003 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated 10... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50401003 (CHEMBL2151197) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 8.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated 10... | J Med Chem 54: 7602-20 (2011) Article DOI: 10.1021/jm200939b BindingDB Entry DOI: 10.7270/Q27D2W9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50296715 (CHEMBL552005 | biphenyl-3-yl 1,4-diazabicyclo[3.2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nAChR expressed in rat GH4C1 cells | Bioorg Med Chem Lett 19: 4747-51 (2009) Article DOI: 10.1016/j.bmcl.2009.06.059 BindingDB Entry DOI: 10.7270/Q21G0N69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309890 (2-(5-Isopropyloxazolo[4,5-b]pyridin-2-yl)-2,5-diaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 229 total ) | Next | Last >> |