

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50558084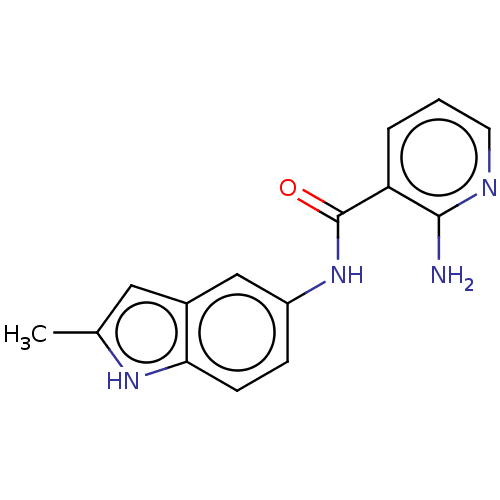 (CHEMBL4799933) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive inhibition of recombinant human full length Avi-tagged p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2(DE3) using 20 uM... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00396 BindingDB Entry DOI: 10.7270/Q2930XVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50558084 (CHEMBL4799933) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of recombinant human full length Avi-tagged p97 (1 to 806 residues) expressed in Escherichia coli Rosetta 2(DE3) using 20 uM A... | Citation and Details Article DOI: 10.1021/acsmedchemlett.5b00396 BindingDB Entry DOI: 10.7270/Q2930XVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282619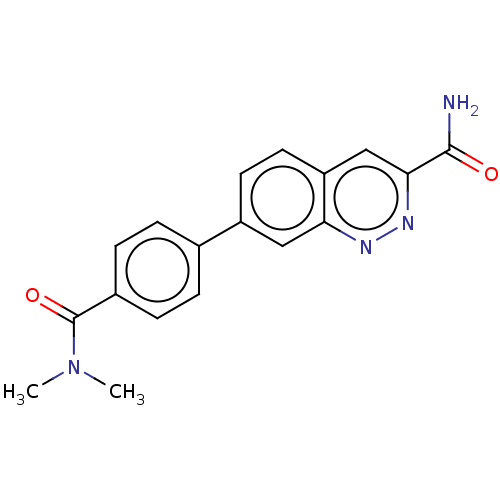 (US9884828, 2-114) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428701 (CHEMBL2333115 | US9884828, 2-127) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... | Bioorg Med Chem Lett 23: 71-4 (2012) Article DOI: 10.1016/j.bmcl.2012.11.021 BindingDB Entry DOI: 10.7270/Q2930VHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428716 (CHEMBL2333128 | US9884828, 2-41) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... | Bioorg Med Chem Lett 23: 71-4 (2012) Article DOI: 10.1016/j.bmcl.2012.11.021 BindingDB Entry DOI: 10.7270/Q2930VHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428717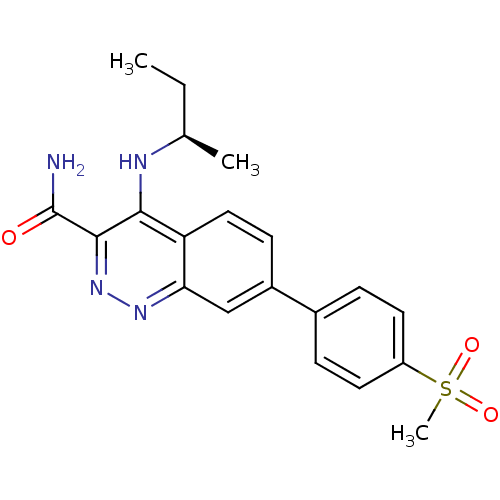 (CHEMBL2333127 | US9884828, 2-37) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... | Bioorg Med Chem Lett 23: 71-4 (2012) Article DOI: 10.1016/j.bmcl.2012.11.021 BindingDB Entry DOI: 10.7270/Q2930VHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282625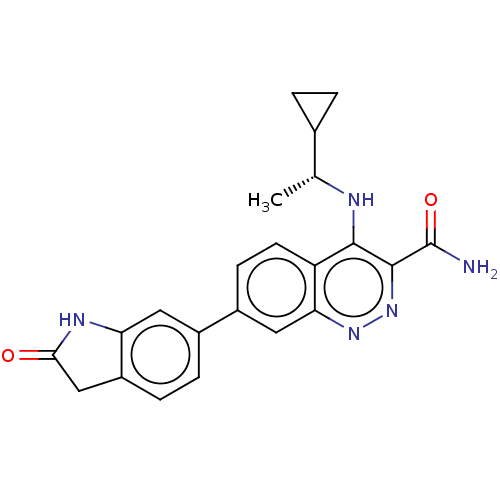 (US9884828, 2-120) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282670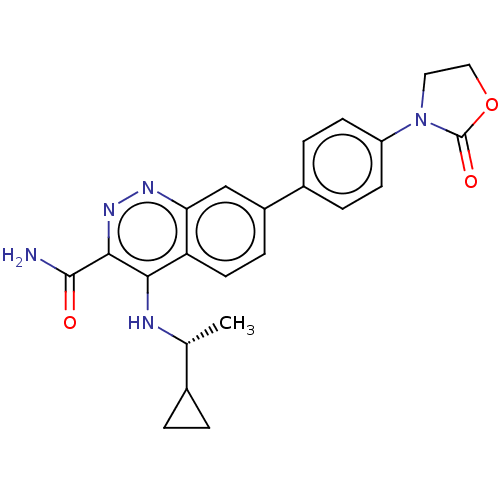 (US9884828, 9-213) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282606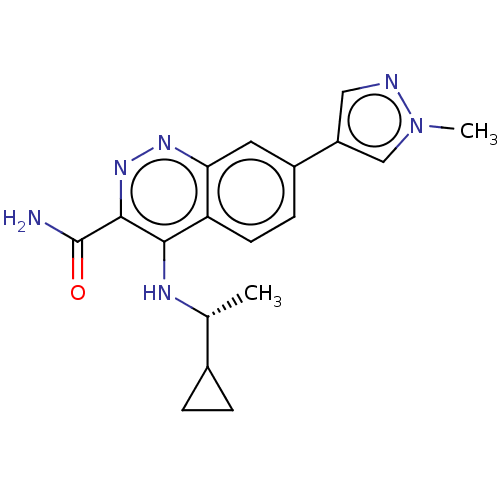 (US9884828, 2-101) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282616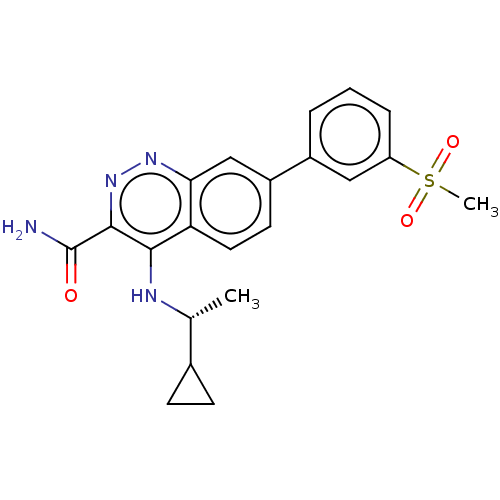 (US9884828, 2-111) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50438363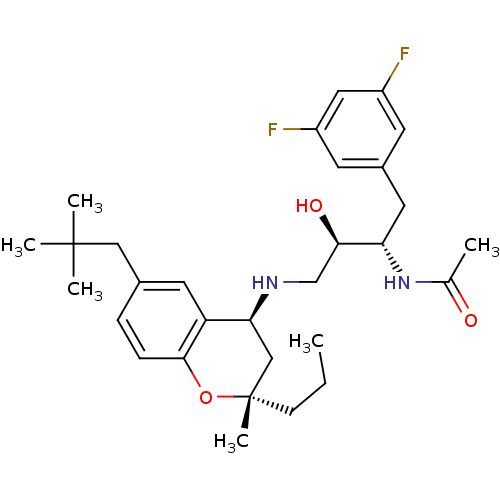 (CHEMBL2408751) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... | Bioorg Med Chem Lett 23: 4674-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.006 BindingDB Entry DOI: 10.7270/Q29W0GWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428703 (CHEMBL2333113 | US9884828, 2-100) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... | Bioorg Med Chem Lett 23: 71-4 (2012) Article DOI: 10.1016/j.bmcl.2012.11.021 BindingDB Entry DOI: 10.7270/Q2930VHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50438362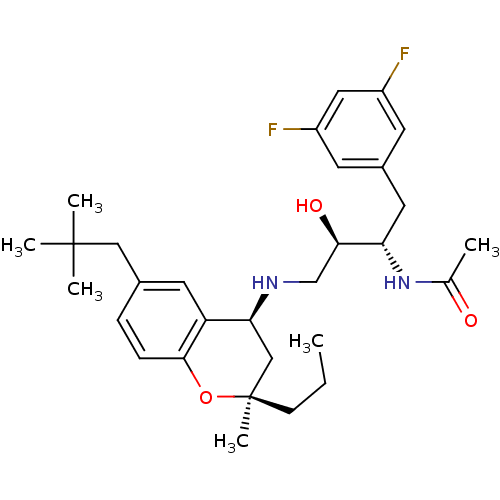 (CHEMBL2408752) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... | Bioorg Med Chem Lett 23: 4674-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.006 BindingDB Entry DOI: 10.7270/Q29W0GWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282626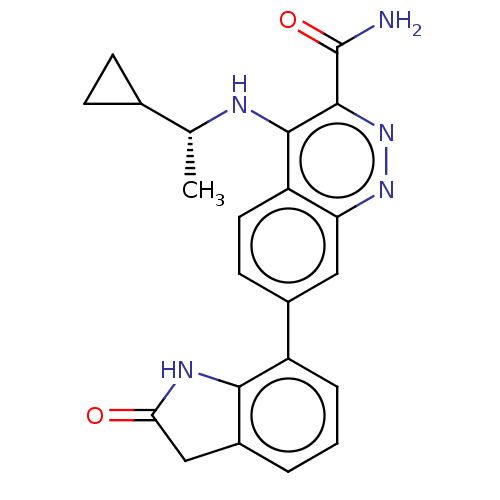 (US9884828, 2-121) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50126127 (CHEMBL3628943) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain using Z-FR-AMC as substrate after 5 mins by fluorescence assay | Bioorg Med Chem Lett 25: 4834-7 (2015) Article DOI: 10.1016/j.bmcl.2015.06.066 BindingDB Entry DOI: 10.7270/Q2H133TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50338292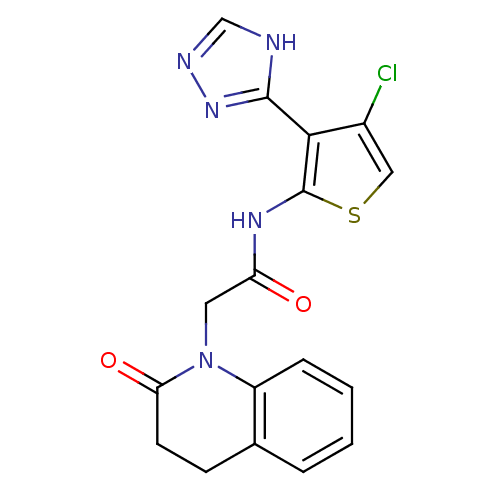 (CHEMBL1682014 | N-(4-chloro-3-(1H-1,2,4-triazol-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JNK1 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 1838-43 (2011) Article DOI: 10.1016/j.bmcl.2011.01.046 BindingDB Entry DOI: 10.7270/Q2Q52PX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50338292 (CHEMBL1682014 | N-(4-chloro-3-(1H-1,2,4-triazol-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JNK3 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 1838-43 (2011) Article DOI: 10.1016/j.bmcl.2011.01.046 BindingDB Entry DOI: 10.7270/Q2Q52PX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50438361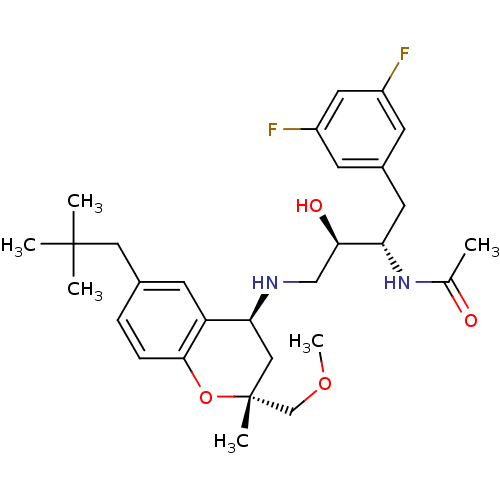 (CHEMBL2408753) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... | Bioorg Med Chem Lett 23: 4674-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.006 BindingDB Entry DOI: 10.7270/Q29W0GWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK2 (Homo sapiens (Human)) | BDBM50436720 (CHEMBL2401963) | PDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of GST-tagged Plk2 (unknown origin) assessed as inhibition of DEKTDDED phosphorylation at Thr1342 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 23: 2743-9 (2013) Article DOI: 10.1016/j.bmcl.2013.02.065 BindingDB Entry DOI: 10.7270/Q28G8N4M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50126138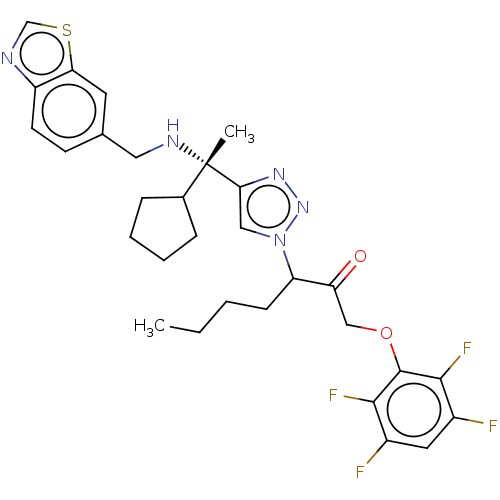 (CHEMBL3628938) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain using Z-FR-AMC as substrate after 5 mins by fluorescence assay | Bioorg Med Chem Lett 25: 4834-7 (2015) Article DOI: 10.1016/j.bmcl.2015.06.066 BindingDB Entry DOI: 10.7270/Q2H133TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50338293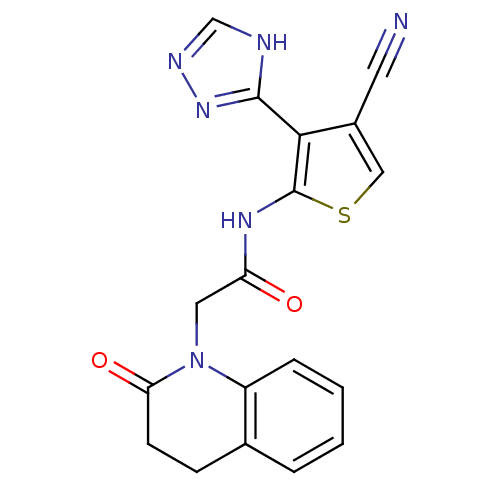 (CHEMBL1682015 | N-(4-cyano-3-(1H-1,2,4-triazol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JNK3 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 1838-43 (2011) Article DOI: 10.1016/j.bmcl.2011.01.046 BindingDB Entry DOI: 10.7270/Q2Q52PX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428701 (CHEMBL2333115 | US9884828, 2-127) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <6 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50438360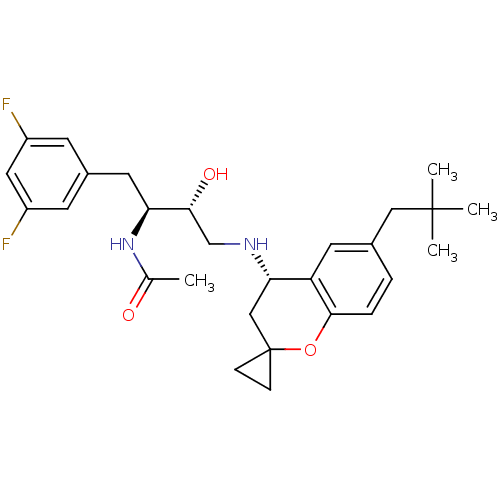 (CHEMBL2408755) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... | Bioorg Med Chem Lett 23: 4674-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.006 BindingDB Entry DOI: 10.7270/Q29W0GWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282617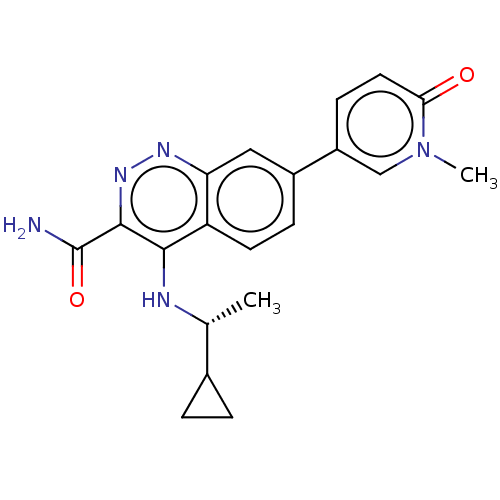 (US9884828, 2-112) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <6 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428716 (CHEMBL2333128 | US9884828, 2-41) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <6 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428703 (CHEMBL2333113 | US9884828, 2-100) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <6 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282607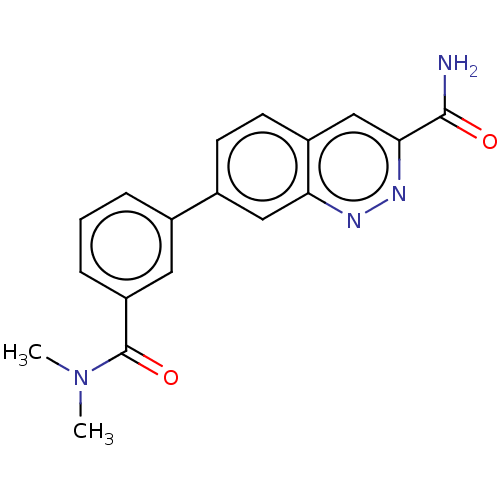 (US9884828, 2-102) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <6 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428717 (CHEMBL2333127 | US9884828, 2-37) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <6 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK2 (Homo sapiens (Human)) | BDBM50436742 (CHEMBL2402081) | PDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of GST-tagged Plk2 (unknown origin) assessed as inhibition of DEKTDDED phosphorylation at Thr1342 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 23: 2743-9 (2013) Article DOI: 10.1016/j.bmcl.2013.02.065 BindingDB Entry DOI: 10.7270/Q28G8N4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50338264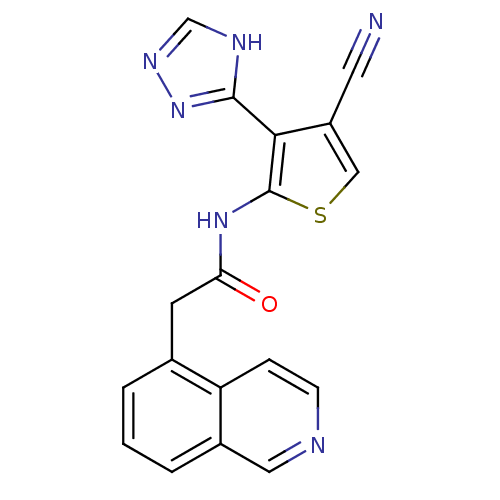 (CHEMBL1682018 | N-(4-cyano-3-(1H-1,2,4-triazol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JNK3 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 1838-43 (2011) Article DOI: 10.1016/j.bmcl.2011.01.046 BindingDB Entry DOI: 10.7270/Q2Q52PX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50338293 (CHEMBL1682015 | N-(4-cyano-3-(1H-1,2,4-triazol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JNK2 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 1838-43 (2011) Article DOI: 10.1016/j.bmcl.2011.01.046 BindingDB Entry DOI: 10.7270/Q2Q52PX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282627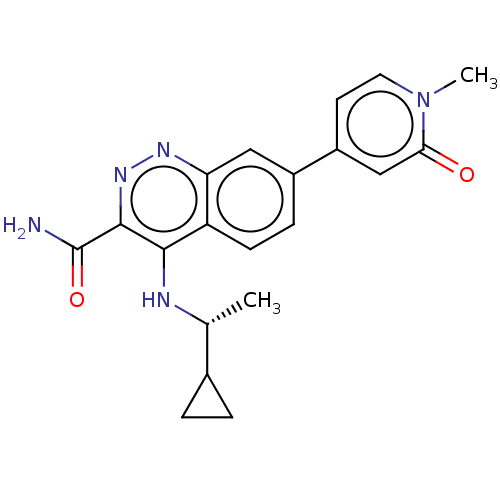 (US9884828, 2-122) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50438358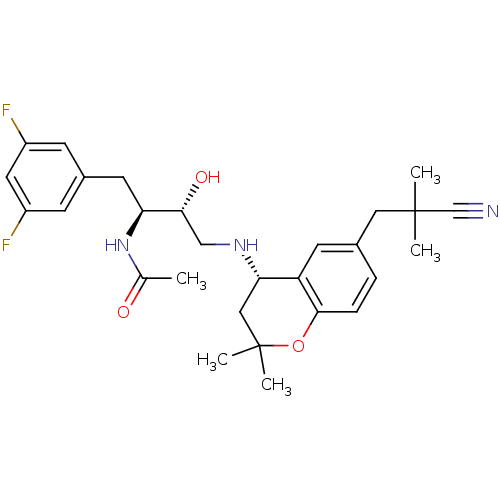 (CHEMBL2408757) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... | Bioorg Med Chem Lett 23: 4674-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.006 BindingDB Entry DOI: 10.7270/Q29W0GWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK2 (Homo sapiens (Human)) | BDBM50436746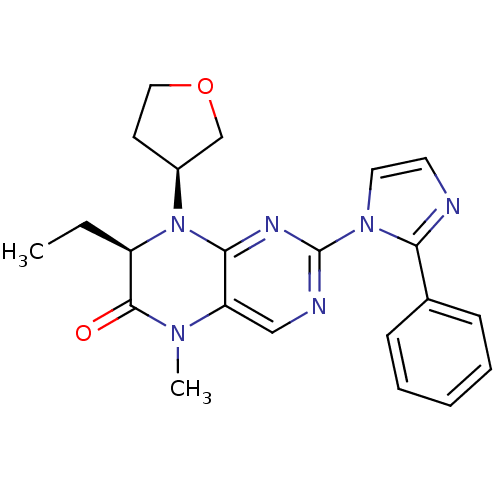 (CHEMBL2401971) | PDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of GST-tagged Plk2 (unknown origin) assessed as inhibition of DEKTDDED phosphorylation at Thr1342 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 23: 2743-9 (2013) Article DOI: 10.1016/j.bmcl.2013.02.065 BindingDB Entry DOI: 10.7270/Q28G8N4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50438359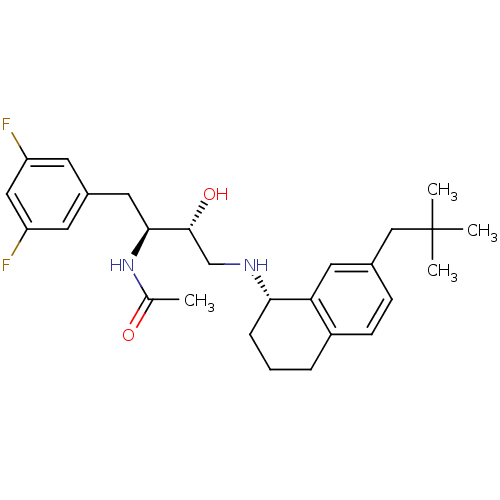 (CHEMBL2408760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... | Bioorg Med Chem Lett 23: 4674-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.006 BindingDB Entry DOI: 10.7270/Q29W0GWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50436720 (CHEMBL2401963) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of GST-tagged Plk1 (unknown origin) assessed as inhibition of DEKTDDED phosphorylation at Thr1342 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 23: 2743-9 (2013) Article DOI: 10.1016/j.bmcl.2013.02.065 BindingDB Entry DOI: 10.7270/Q28G8N4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428696 (CHEMBL2333120) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... | Bioorg Med Chem Lett 23: 71-4 (2012) Article DOI: 10.1016/j.bmcl.2012.11.021 BindingDB Entry DOI: 10.7270/Q2930VHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428698 (CHEMBL2333118 | US9884828, 2-35) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... | Bioorg Med Chem Lett 23: 71-4 (2012) Article DOI: 10.1016/j.bmcl.2012.11.021 BindingDB Entry DOI: 10.7270/Q2930VHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50338292 (CHEMBL1682014 | N-(4-chloro-3-(1H-1,2,4-triazol-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JNK2 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 1838-43 (2011) Article DOI: 10.1016/j.bmcl.2011.01.046 BindingDB Entry DOI: 10.7270/Q2Q52PX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK2 (Homo sapiens (Human)) | BDBM50436734 (CHEMBL2402079) | PDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of GST-tagged Plk2 (unknown origin) assessed as inhibition of DEKTDDED phosphorylation at Thr1342 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 23: 2743-9 (2013) Article DOI: 10.1016/j.bmcl.2013.02.065 BindingDB Entry DOI: 10.7270/Q28G8N4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428700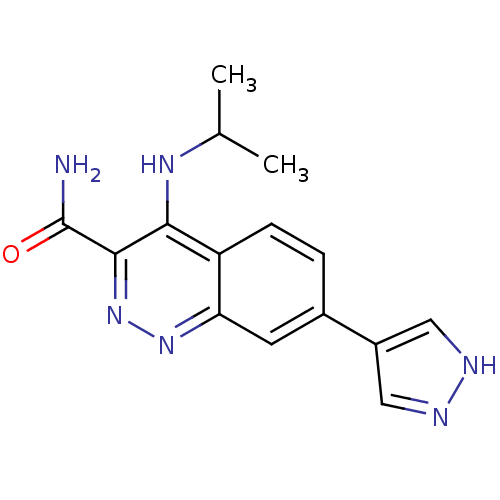 (CHEMBL2333116 | US9884828, 2-53) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50338293 (CHEMBL1682015 | N-(4-cyano-3-(1H-1,2,4-triazol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JNK1 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 21: 1838-43 (2011) Article DOI: 10.1016/j.bmcl.2011.01.046 BindingDB Entry DOI: 10.7270/Q2Q52PX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282674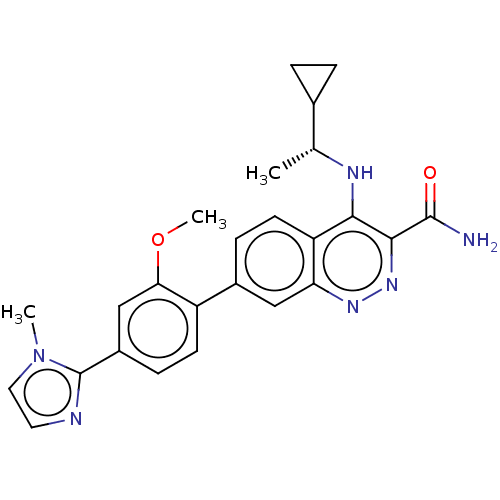 (US9884828, 11-221) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428698 (CHEMBL2333118 | US9884828, 2-35) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282589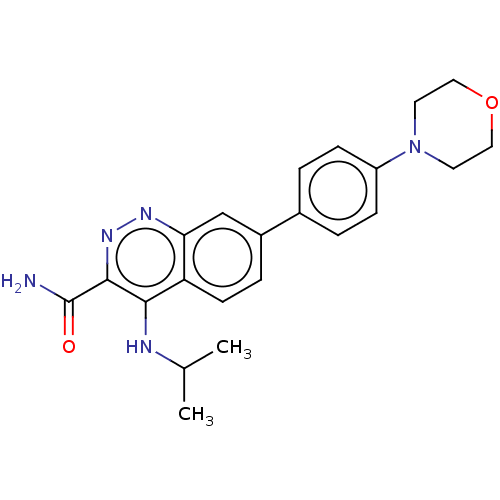 (US9884828, 2-49) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK2 (Homo sapiens (Human)) | BDBM50436749 (CHEMBL2401966) | PDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of GST-tagged Plk2 (unknown origin) assessed as inhibition of DEKTDDED phosphorylation at Thr1342 after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 23: 2743-9 (2013) Article DOI: 10.1016/j.bmcl.2013.02.065 BindingDB Entry DOI: 10.7270/Q28G8N4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428715 (CHEMBL2333129 | US9884828, 2-19) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... | Bioorg Med Chem Lett 23: 71-4 (2012) Article DOI: 10.1016/j.bmcl.2012.11.021 BindingDB Entry DOI: 10.7270/Q2930VHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50428715 (CHEMBL2333129 | US9884828, 2-19) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282623 (US9884828, 2-118) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM282621 (US9884828, 2-116) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc. US Patent | Assay Description Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... | US Patent US9884828 (2018) BindingDB Entry DOI: 10.7270/Q2NG4SNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1406 total ) | Next | Last >> |