

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532854 (CHEMBL4588569) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532854 (CHEMBL4588569) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532843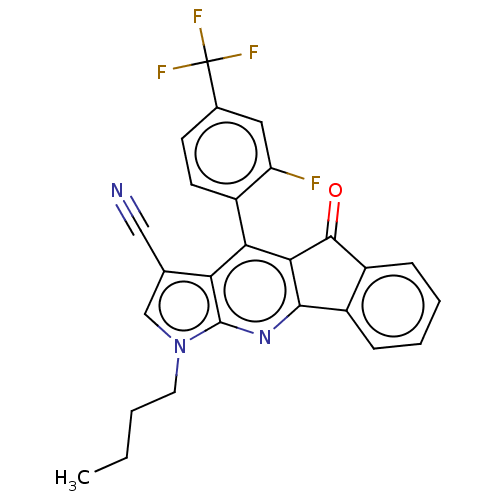 (CHEMBL4545462) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532843 (CHEMBL4545462) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using A5'-end 32P-labeled 24-mer primer/a48-mer template preincubated for 5 mins followed by 25 uM nucleotides addition measure... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using A5'-end 32P-labeled 24-mer primer/a48-mer template preincubated for 5 mins followed by 25 uM nucleotides addition measure... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using A5'-end 32P-labeled 24-mer primer/a48-mer template preincubated for 5 mins followed by 10 uM nucleotides addition measure... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using A5'-end 32P-labeled 24-mer primer/a48-mer template preincubated for 5 mins followed by 10 uM nucleotides addition measure... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532846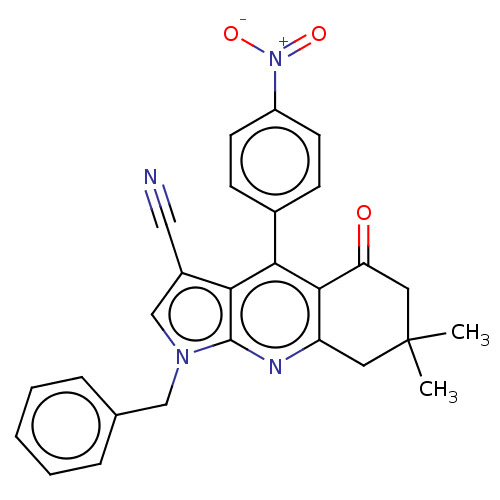 (CHEMBL4533325) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532846 (CHEMBL4533325) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50432235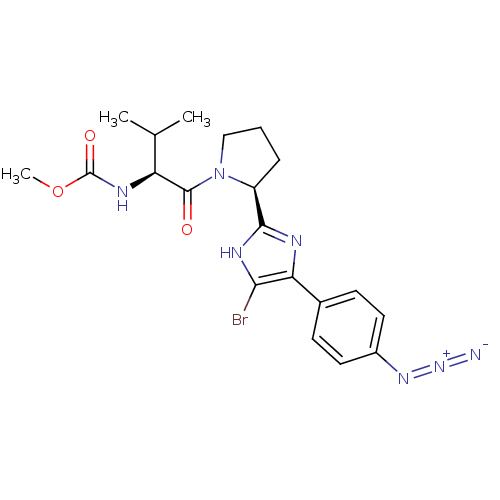 (CHEMBL2347323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 | Bioorg Med Chem Lett 23: 2031-4 (2013) Article DOI: 10.1016/j.bmcl.2013.02.023 BindingDB Entry DOI: 10.7270/Q2833TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532849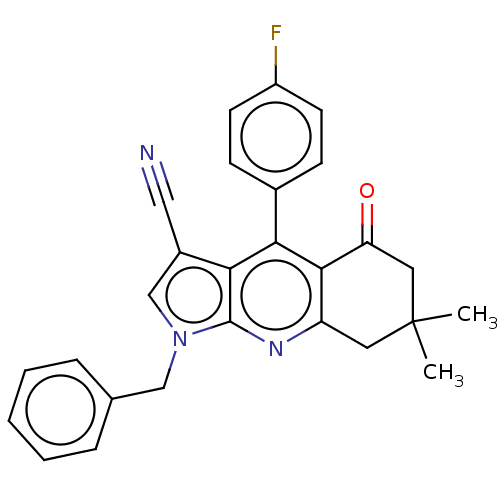 (CHEMBL4592520) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532849 (CHEMBL4592520) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT Y181C mutant using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincub... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT Y181C mutant using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincub... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50432236 (CHEMBL2347159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 | Bioorg Med Chem Lett 23: 2031-4 (2013) Article DOI: 10.1016/j.bmcl.2013.02.023 BindingDB Entry DOI: 10.7270/Q2833TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532848 (CHEMBL4443785) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532848 (CHEMBL4443785) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50432234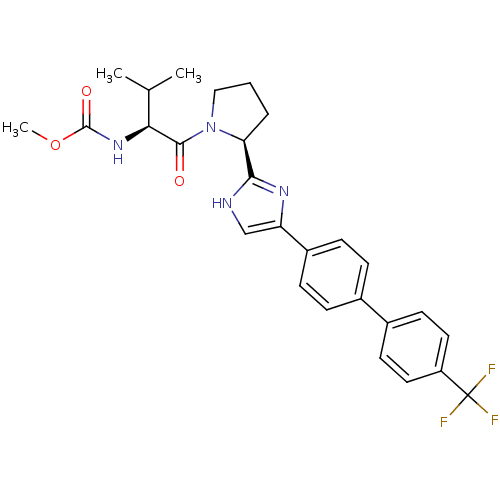 (CHEMBL2347328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 | Bioorg Med Chem Lett 23: 2031-4 (2013) Article DOI: 10.1016/j.bmcl.2013.02.023 BindingDB Entry DOI: 10.7270/Q2833TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532854 (CHEMBL4588569) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT Y181C mutant using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincub... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532854 (CHEMBL4588569) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT Y181C mutant using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincub... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50432233 (CHEMBL2347337) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 | Bioorg Med Chem Lett 23: 2031-4 (2013) Article DOI: 10.1016/j.bmcl.2013.02.023 BindingDB Entry DOI: 10.7270/Q2833TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50387084 (BMS-790052 | DACLATASVIR) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 | Bioorg Med Chem Lett 23: 2031-4 (2013) Article DOI: 10.1016/j.bmcl.2013.02.023 BindingDB Entry DOI: 10.7270/Q2833TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using A5'-end 32P-labeled 24-mer primer/a48-mer template preincubated for 5 mins followed by 1 uM nucleotides addition measured... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using A5'-end 32P-labeled 24-mer primer/a48-mer template preincubated for 5 mins followed by 1 uM nucleotides addition measured... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50432234 (CHEMBL2347328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 | Bioorg Med Chem Lett 23: 2031-4 (2013) Article DOI: 10.1016/j.bmcl.2013.02.023 BindingDB Entry DOI: 10.7270/Q2833TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT K103N mutant using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincub... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT K103N mutant using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincub... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using A5'-end 32P-labeled 24-mer primer/a48-mer template preincubated for 5 mins followed by 0.5 uM nucleotides addition measur... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532847 (CHEMBL4529463) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using A5'-end 32P-labeled 24-mer primer/a48-mer template preincubated for 5 mins followed by 0.5 uM nucleotides addition measur... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532857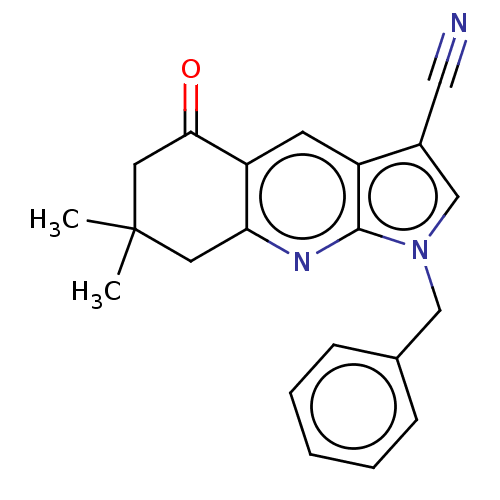 (CHEMBL4459763) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532857 (CHEMBL4459763) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532845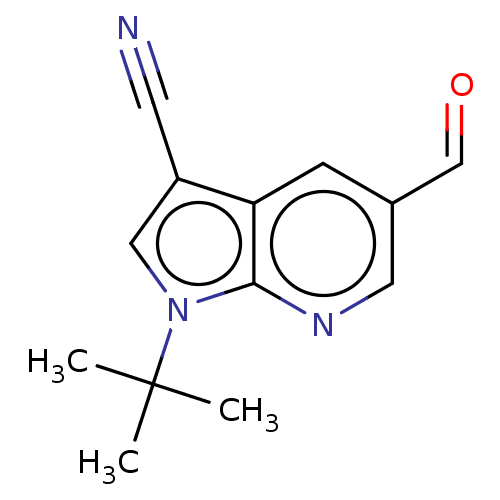 (CHEMBL4532532) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532845 (CHEMBL4532532) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532853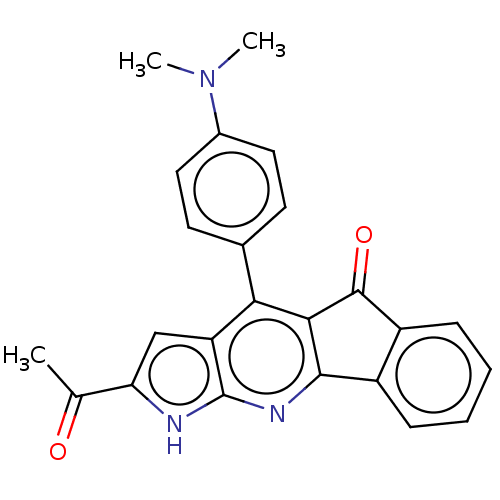 (CHEMBL4475411) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532848 (CHEMBL4443785) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT K103N mutant using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincub... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532853 (CHEMBL4475411) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532848 (CHEMBL4443785) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT K103N mutant using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincub... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532856 (CHEMBL4577153) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532856 (CHEMBL4577153) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincubated for 10 m... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532843 (CHEMBL4545462) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT K103N mutant using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincub... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50532843 (CHEMBL4545462) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of HIV1 RT K103N mutant using 17-mer DNA/Alexa Fluor 488 5'-end labeled DNA/Alexa Fluor 555-aha-dUTP as primer/template/substrate preincub... | Bioorg Med Chem Lett 26: 4101-5 (2016) Article DOI: 10.1016/j.bmcl.2016.06.065 BindingDB Entry DOI: 10.7270/Q27S7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50432233 (CHEMBL2347337) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 | Bioorg Med Chem Lett 23: 2031-4 (2013) Article DOI: 10.1016/j.bmcl.2013.02.023 BindingDB Entry DOI: 10.7270/Q2833TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50432235 (CHEMBL2347323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 | Bioorg Med Chem Lett 23: 2031-4 (2013) Article DOI: 10.1016/j.bmcl.2013.02.023 BindingDB Entry DOI: 10.7270/Q2833TDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 105 total ) | Next | Last >> |