 Found 141 hits with Last Name = 'nikiforovich' and Initial = 'g'
Found 141 hits with Last Name = 'nikiforovich' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor B
(RAT) | BDBM50015662
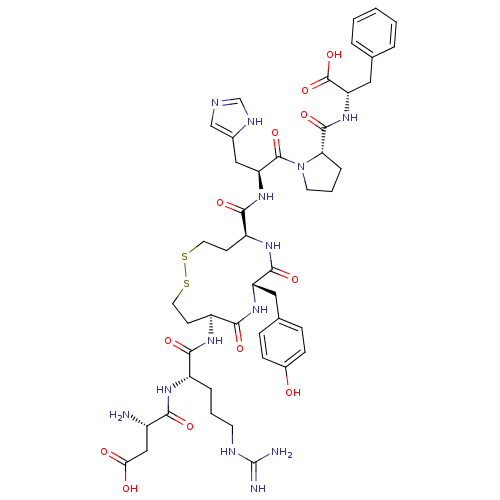
(3-Amino-N-{1-[5-[2-[2-(1-carboxy-2-phenyl-ethylcar...)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CCSSCC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C47H63N13O12S2/c48-30(23-38(62)63)39(64)54-31(8-4-16-52-47(49)50)40(65)55-32-14-18-73-74-19-15-33(56-43(68)34(57-41(32)66)20-27-10-12-29(61)13-11-27)42(67)58-35(22-28-24-51-25-53-28)45(70)60-17-5-9-37(60)44(69)59-36(46(71)72)21-26-6-2-1-3-7-26/h1-3,6-7,10-13,24-25,30-37,61H,4-5,8-9,14-23,48H2,(H,51,53)(H,54,64)(H,55,65)(H,56,68)(H,57,66)(H,58,67)(H,59,69)(H,62,63)(H,71,72)(H4,49,50,52)/t30-,31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity at rat liver Angiotensin II receptor, type 1 was determined based on displacement of [125I]-Ang II |
J Med Chem 45: 1767-77 (2002)
BindingDB Entry DOI: 10.7270/Q2QV3N7W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50112098
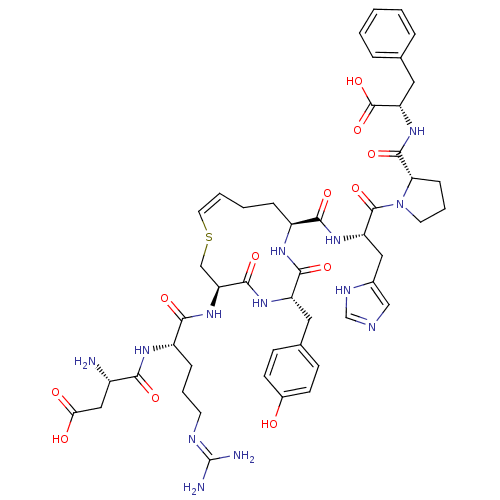
(CHEMBL412045 | analog of Angiotensin II with cis v...)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CS\C=C/CC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |c:23| Show InChI InChI=1S/C48H63N13O12S/c49-31(23-39(63)64)40(65)55-33(11-6-17-53-48(50)51)42(67)60-37-25-74-19-5-4-10-32(56-43(68)34(57-44(37)69)20-28-13-15-30(62)16-14-28)41(66)58-35(22-29-24-52-26-54-29)46(71)61-18-7-12-38(61)45(70)59-36(47(72)73)21-27-8-2-1-3-9-27/h1-3,5,8-9,13-16,19,24,26,31-38,62H,4,6-7,10-12,17-18,20-23,25,49H2,(H,52,54)(H,55,65)(H,56,68)(H,57,69)(H,58,66)(H,59,70)(H,60,67)(H,63,64)(H,72,73)(H4,50,51,53)/b19-5-/t31-,32-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity at rat liver Angiotensin II receptor, type 1 was determined based on displacement of [125I]-Ang II |
J Med Chem 45: 1767-77 (2002)
BindingDB Entry DOI: 10.7270/Q2QV3N7W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50112097
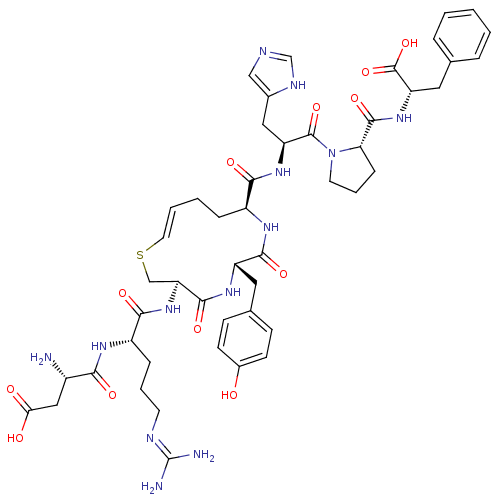
(CHEMBL266450 | analog of Angiotensin II with trans...)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]1CS\C=C\CC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |t:23| Show InChI InChI=1S/C48H63N13O12S/c49-31(23-39(63)64)40(65)55-33(11-6-17-53-48(50)51)42(67)60-37-25-74-19-5-4-10-32(56-43(68)34(57-44(37)69)20-28-13-15-30(62)16-14-28)41(66)58-35(22-29-24-52-26-54-29)46(71)61-18-7-12-38(61)45(70)59-36(47(72)73)21-27-8-2-1-3-9-27/h1-3,5,8-9,13-16,19,24,26,31-38,62H,4,6-7,10-12,17-18,20-23,25,49H2,(H,52,54)(H,55,65)(H,56,68)(H,57,69)(H,58,66)(H,59,70)(H,60,67)(H,63,64)(H,72,73)(H4,50,51,53)/b19-5+/t31-,32-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
In vitro binding affinity at rat liver Angiotensin II receptor, type 1 was determined based on displacement of [125I]-Ang II |
J Med Chem 45: 1767-77 (2002)
BindingDB Entry DOI: 10.7270/Q2QV3N7W |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50001468
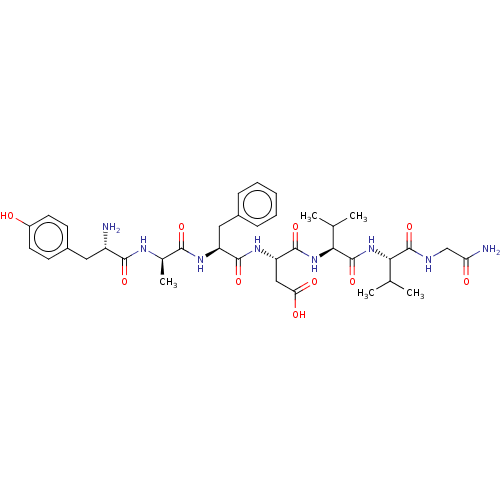
((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C37H52N8O10/c1-19(2)30(36(54)40-18-28(39)47)45-37(55)31(20(3)4)44-35(53)27(17-29(48)49)43-34(52)26(16-22-9-7-6-8-10-22)42-32(50)21(5)41-33(51)25(38)15-23-11-13-24(46)14-12-23/h6-14,19-21,25-27,30-31,46H,15-18,38H2,1-5H3,(H2,39,47)(H,40,54)(H,41,51)(H,42,50)(H,43,52)(H,44,53)(H,45,55)(H,48,49)/t21-,25+,26+,27+,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mouse vas deferens smooth muscle |
Bioorg Med Chem Lett 2: 547-552 (1992)
Article DOI: 10.1016/S0960-894X(01)81195-1
BindingDB Entry DOI: 10.7270/Q23R0STD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50280456
(CHEMBL153466 | {(4S,7R,10S,13R)-13-[(S)-2-Amino-3-...)Show SMILES CC(C)[C@H](NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CC(O)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C35H46N8O10S2/c1-18(2)29(35(53)38-15-27(37)45)43-34(52)26-17-55-54-16-25(41-30(48)22(36)12-20-8-10-21(44)11-9-20)33(51)39-23(13-19-6-4-3-5-7-19)31(49)40-24(14-28(46)47)32(50)42-26/h3-11,18,22-26,29,44H,12-17,36H2,1-2H3,(H2,37,45)(H,38,53)(H,39,51)(H,40,49)(H,41,48)(H,42,50)(H,43,52)(H,46,47)/t22-,23-,24+,25-,26+,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mouse vas deferens smooth muscle |
Bioorg Med Chem Lett 2: 547-552 (1992)
Article DOI: 10.1016/S0960-894X(01)81195-1
BindingDB Entry DOI: 10.7270/Q23R0STD |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50184359
((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 1 receptor (hMC1R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50454555
(CHEMBL2115441)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O |wU:30.30,66.69,4.4,44.46,25.25,wD:12.11,21.80,55.57,(-3.6,-3.27,;-2.8,-4.62,;-3.51,-6.01,;-2.66,-7.31,;-3.24,-8.75,;-4.77,-8.95,;-5.17,-10.44,;-6.68,-10.84,;-4.09,-11.55,;-2.3,-9.96,;-2.87,-11.38,;-.49,-9.48,;.9,-10.13,;.41,-11.59,;.29,-13.13,;-1.26,-13.19,;.53,-14.65,;1.14,-16.07,;2.06,-17.29,;3.26,-18.26,;4.65,-18.92,;6.15,-19.23,;7.69,-19.18,;9.17,-18.75,;9.77,-20.16,;10.5,-17.98,;11.43,-19.2,;11.62,-16.91,;12.44,-15.6,;13.83,-16.26,;12.93,-14.14,;14.44,-14.44,;14.92,-15.92,;16.39,-16.38,;16.4,-17.92,;14.94,-18.4,;14.33,-19.81,;12.8,-19.99,;11.88,-18.75,;12.49,-17.34,;14.02,-17.17,;13.05,-12.61,;12.8,-11.09,;14.28,-10.67,;12.2,-9.68,;13.53,-8.91,;14.87,-9.68,;16.19,-8.91,;17.53,-9.68,;18.87,-8.91,;20.19,-9.68,;18.87,-7.37,;11.27,-8.44,;10.09,-7.47,;10.9,-6.17,;8.69,-6.81,;9.17,-5.35,;10.69,-5.05,;11.17,-3.59,;12.68,-3.28,;13.71,-4.44,;13.22,-5.89,;11.7,-6.19,;7.18,-6.51,;5.64,-6.55,;5.92,-5.52,;4.17,-6.99,;3.56,-5.58,;4.48,-4.34,;6.03,-4.38,;6.52,-2.9,;5.3,-1.99,;4.03,-2.88,;2.83,-7.77,;1.72,-8.83,;.53,-7.85,;6.03,-20.77,;7.29,-21.65,;4.64,-21.43,)| Show InChI InChI=1S/C53H74N16O10/c1-4-5-17-38(63-31(3)70)47(74)69-43-26-44(71)58-21-12-11-19-37(45(54)72)64-46(73)30(2)62-49(76)41(24-33-27-60-36-18-10-9-16-35(33)36)67-48(75)39(20-13-22-59-53(55)56)65-50(77)40(23-32-14-7-6-8-15-32)66-51(78)42(68-52(43)79)25-34-28-57-29-61-34/h6-10,14-16,18,27-30,37-43,60H,4-5,11-13,17,19-26H2,1-3H3,(H2,54,72)(H,57,61)(H,58,71)(H,62,76)(H,63,70)(H,64,73)(H,65,77)(H,66,78)(H,67,75)(H,68,79)(H,69,74)(H4,55,56,59)/t30-,37-,38-,39-,40+,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 1 receptor (hMC1R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50035820
(Ac-Trp-L-3-MPt(Pr)-Asp-Phe-NH2 | CHEMBL37830)Show SMILES CCCS[C@@H]1CCN([C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O Show InChI InChI=1S/C34H42N6O7S/c1-3-15-48-28-13-14-40(34(47)27(37-20(2)41)17-22-19-36-24-12-8-7-11-23(22)24)30(28)33(46)39-26(18-29(42)43)32(45)38-25(31(35)44)16-21-9-5-4-6-10-21/h4-12,19,25-28,30,36H,3,13-18H2,1-2H3,(H2,35,44)(H,37,41)(H,38,45)(H,39,46)(H,42,43)/t25-,26-,27-,28+,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells |
J Med Chem 38: 137-49 (1995)
BindingDB Entry DOI: 10.7270/Q2QN65TS |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM82411
(CAS_75921-69-6 | NDP-MSH)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:66.68,55.56,45.45,111.116,4.4,14.23,wD:77.79,95.99,107.113,36.36,8.10,26.29,(-7.66,4.36,;-8.98,3.65,;-9.15,2.12,;-10.5,1.37,;-10.5,-.23,;-11.93,-.94,;-13.21,-.15,;-13.17,1.41,;-14.6,-.83,;-14.64,-2.37,;-13.43,-3.18,;-15.78,-.09,;-17.19,-.68,;-17.34,-2.22,;-18.55,.02,;-18.4,1.67,;-17.08,2.44,;-17.08,3.97,;-15.61,4.72,;-14.35,3.82,;-13,4.55,;-14.45,2.26,;-15.78,1.52,;-19.9,-.62,;-21.14,.17,;-21.14,1.73,;-22.53,-.62,;-22.53,-2.12,;-21.33,-2.88,;-23.81,.17,;-25.13,-.41,;-26.27,.45,;-25.2,-1.9,;-9.26,-1,;-9.3,-2.5,;-7.94,-.34,;-6.59,-1.15,;-6.59,-2.71,;-7.94,-3.46,;-7.94,-5,;-9.3,-5.6,;-6.63,-5.81,;-5.24,-.34,;-5.2,1.15,;-3.86,-1.11,;-2.57,-.23,;-2.57,1.26,;-1.19,2.05,;.1,1.47,;1.29,2.58,;.52,3.87,;-.97,3.65,;-1.19,-1,;-1.19,-2.5,;.1,-.3,;1.55,-1.15,;1.55,-2.65,;2.79,-3.4,;2.79,-4.89,;4.22,-5.7,;5.56,-4.89,;5.56,-3.4,;4.22,-2.65,;2.79,-.34,;2.79,1.2,;4.22,-1,;5.33,-.09,;5.29,1.47,;6.63,2.26,;6.53,3.82,;7.81,4.72,;7.81,6.13,;9.13,7.03,;6.46,6.92,;6.85,-.73,;7,-2.37,;8,.13,;9.35,-.41,;9.69,-2.07,;10.95,-2.71,;12.29,-1.9,;13.51,-2.99,;12.83,-4.36,;13.43,-5.81,;12.55,-7.03,;10.95,-6.77,;10.33,-5.43,;11.29,-4.21,;10.69,.41,;10.48,2.16,;12.04,-.41,;13.38,.3,;14.58,-.51,;14.47,-2.03,;15.99,.17,;17.25,-.68,;17.25,-2.26,;18.49,-3.08,;18.45,-4.57,;19.73,-5.55,;19.69,-7.03,;18.66,.02,;18.77,1.52,;20.01,-.68,;20.09,-2.22,;21.72,-2.54,;22.29,-1.11,;21.5,-.09,;21.78,1.47,;20.65,2.84,;23.32,1.64,;23.83,2.97,;23,4.19,;23.6,5.58,;21.5,4.04,;25.41,3.12,;26.03,4.44,;26.27,1.94,)| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 1 receptor (hMC1R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50001468

((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C37H52N8O10/c1-19(2)30(36(54)40-18-28(39)47)45-37(55)31(20(3)4)44-35(53)27(17-29(48)49)43-34(52)26(16-22-9-7-6-8-10-22)42-32(50)21(5)41-33(51)25(38)15-23-11-13-24(46)14-12-23/h6-14,19-21,25-27,30-31,46H,15-18,38H2,1-5H3,(H2,39,47)(H,40,54)(H,41,51)(H,42,50)(H,43,52)(H,44,53)(H,45,55)(H,48,49)/t21-,25+,26+,27+,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against delta receptor with [3H]-[p-Cl-Phe4]-DPDPE |
Bioorg Med Chem Lett 2: 547-552 (1992)
Article DOI: 10.1016/S0960-894X(01)81195-1
BindingDB Entry DOI: 10.7270/Q23R0STD |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 4 receptor (hMC4R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21146

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-[(3S)-3-...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C55H66N10O13/c1-3-4-19-45(54(77)64-43(29-48(70)71)53(76)61-40(49(57)72)24-32-13-7-5-8-14-32)65(2)55(78)44(27-35-30-58-39-18-12-11-17-37(35)39)60-46(67)31-59-51(74)41(25-33-15-9-6-10-16-33)63-52(75)42(26-34-20-22-36(66)23-21-34)62-50(73)38(56)28-47(68)69/h5-18,20-23,30,38,40-45,58,66H,3-4,19,24-29,31,56H2,1-2H3,(H2,57,72)(H,59,74)(H,60,67)(H,61,76)(H,62,73)(H,63,75)(H,64,77)(H,68,69)(H,70,71)/t38-,40-,41+,42-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor was determined in guinea pig cortex using [3H]SNF8702 as radioligand |
J Med Chem 39: 4120-4 (1996)
Article DOI: 10.1021/jm960078j
BindingDB Entry DOI: 10.7270/Q2P55P5Z |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM82411

(CAS_75921-69-6 | NDP-MSH)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:66.68,55.56,45.45,111.116,4.4,14.23,wD:77.79,95.99,107.113,36.36,8.10,26.29,(-7.66,4.36,;-8.98,3.65,;-9.15,2.12,;-10.5,1.37,;-10.5,-.23,;-11.93,-.94,;-13.21,-.15,;-13.17,1.41,;-14.6,-.83,;-14.64,-2.37,;-13.43,-3.18,;-15.78,-.09,;-17.19,-.68,;-17.34,-2.22,;-18.55,.02,;-18.4,1.67,;-17.08,2.44,;-17.08,3.97,;-15.61,4.72,;-14.35,3.82,;-13,4.55,;-14.45,2.26,;-15.78,1.52,;-19.9,-.62,;-21.14,.17,;-21.14,1.73,;-22.53,-.62,;-22.53,-2.12,;-21.33,-2.88,;-23.81,.17,;-25.13,-.41,;-26.27,.45,;-25.2,-1.9,;-9.26,-1,;-9.3,-2.5,;-7.94,-.34,;-6.59,-1.15,;-6.59,-2.71,;-7.94,-3.46,;-7.94,-5,;-9.3,-5.6,;-6.63,-5.81,;-5.24,-.34,;-5.2,1.15,;-3.86,-1.11,;-2.57,-.23,;-2.57,1.26,;-1.19,2.05,;.1,1.47,;1.29,2.58,;.52,3.87,;-.97,3.65,;-1.19,-1,;-1.19,-2.5,;.1,-.3,;1.55,-1.15,;1.55,-2.65,;2.79,-3.4,;2.79,-4.89,;4.22,-5.7,;5.56,-4.89,;5.56,-3.4,;4.22,-2.65,;2.79,-.34,;2.79,1.2,;4.22,-1,;5.33,-.09,;5.29,1.47,;6.63,2.26,;6.53,3.82,;7.81,4.72,;7.81,6.13,;9.13,7.03,;6.46,6.92,;6.85,-.73,;7,-2.37,;8,.13,;9.35,-.41,;9.69,-2.07,;10.95,-2.71,;12.29,-1.9,;13.51,-2.99,;12.83,-4.36,;13.43,-5.81,;12.55,-7.03,;10.95,-6.77,;10.33,-5.43,;11.29,-4.21,;10.69,.41,;10.48,2.16,;12.04,-.41,;13.38,.3,;14.58,-.51,;14.47,-2.03,;15.99,.17,;17.25,-.68,;17.25,-2.26,;18.49,-3.08,;18.45,-4.57,;19.73,-5.55,;19.69,-7.03,;18.66,.02,;18.77,1.52,;20.01,-.68,;20.09,-2.22,;21.72,-2.54,;22.29,-1.11,;21.5,-.09,;21.78,1.47,;20.65,2.84,;23.32,1.64,;23.83,2.97,;23,4.19,;23.6,5.58,;21.5,4.04,;25.41,3.12,;26.03,4.44,;26.27,1.94,)| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 5 receptor (hMC5R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50280456

(CHEMBL153466 | {(4S,7R,10S,13R)-13-[(S)-2-Amino-3-...)Show SMILES CC(C)[C@H](NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CC(O)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C35H46N8O10S2/c1-18(2)29(35(53)38-15-27(37)45)43-34(52)26-17-55-54-16-25(41-30(48)22(36)12-20-8-10-21(44)11-9-20)33(51)39-23(13-19-6-4-3-5-7-19)31(49)40-24(14-28(46)47)32(50)42-26/h3-11,18,22-26,29,44H,12-17,36H2,1-2H3,(H2,37,45)(H,38,53)(H,39,51)(H,40,49)(H,41,48)(H,42,50)(H,43,52)(H,46,47)/t22-,23-,24+,25-,26+,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against delta receptor with [3H]-[p-Cl-Phe4]-DPDPE |
Bioorg Med Chem Lett 2: 547-552 (1992)
Article DOI: 10.1016/S0960-894X(01)81195-1
BindingDB Entry DOI: 10.7270/Q23R0STD |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50454555

(CHEMBL2115441)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O |wU:30.30,66.69,4.4,44.46,25.25,wD:12.11,21.80,55.57,(-3.6,-3.27,;-2.8,-4.62,;-3.51,-6.01,;-2.66,-7.31,;-3.24,-8.75,;-4.77,-8.95,;-5.17,-10.44,;-6.68,-10.84,;-4.09,-11.55,;-2.3,-9.96,;-2.87,-11.38,;-.49,-9.48,;.9,-10.13,;.41,-11.59,;.29,-13.13,;-1.26,-13.19,;.53,-14.65,;1.14,-16.07,;2.06,-17.29,;3.26,-18.26,;4.65,-18.92,;6.15,-19.23,;7.69,-19.18,;9.17,-18.75,;9.77,-20.16,;10.5,-17.98,;11.43,-19.2,;11.62,-16.91,;12.44,-15.6,;13.83,-16.26,;12.93,-14.14,;14.44,-14.44,;14.92,-15.92,;16.39,-16.38,;16.4,-17.92,;14.94,-18.4,;14.33,-19.81,;12.8,-19.99,;11.88,-18.75,;12.49,-17.34,;14.02,-17.17,;13.05,-12.61,;12.8,-11.09,;14.28,-10.67,;12.2,-9.68,;13.53,-8.91,;14.87,-9.68,;16.19,-8.91,;17.53,-9.68,;18.87,-8.91,;20.19,-9.68,;18.87,-7.37,;11.27,-8.44,;10.09,-7.47,;10.9,-6.17,;8.69,-6.81,;9.17,-5.35,;10.69,-5.05,;11.17,-3.59,;12.68,-3.28,;13.71,-4.44,;13.22,-5.89,;11.7,-6.19,;7.18,-6.51,;5.64,-6.55,;5.92,-5.52,;4.17,-6.99,;3.56,-5.58,;4.48,-4.34,;6.03,-4.38,;6.52,-2.9,;5.3,-1.99,;4.03,-2.88,;2.83,-7.77,;1.72,-8.83,;.53,-7.85,;6.03,-20.77,;7.29,-21.65,;4.64,-21.43,)| Show InChI InChI=1S/C53H74N16O10/c1-4-5-17-38(63-31(3)70)47(74)69-43-26-44(71)58-21-12-11-19-37(45(54)72)64-46(73)30(2)62-49(76)41(24-33-27-60-36-18-10-9-16-35(33)36)67-48(75)39(20-13-22-59-53(55)56)65-50(77)40(23-32-14-7-6-8-15-32)66-51(78)42(68-52(43)79)25-34-28-57-29-61-34/h6-10,14-16,18,27-30,37-43,60H,4-5,11-13,17,19-26H2,1-3H3,(H2,54,72)(H,57,61)(H,58,71)(H,62,76)(H,63,70)(H,64,73)(H,65,77)(H,66,78)(H,67,75)(H,68,79)(H,69,74)(H4,55,56,59)/t30-,37-,38-,39-,40+,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 4 receptor (hMC4R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM82411

(CAS_75921-69-6 | NDP-MSH)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:66.68,55.56,45.45,111.116,4.4,14.23,wD:77.79,95.99,107.113,36.36,8.10,26.29,(-7.66,4.36,;-8.98,3.65,;-9.15,2.12,;-10.5,1.37,;-10.5,-.23,;-11.93,-.94,;-13.21,-.15,;-13.17,1.41,;-14.6,-.83,;-14.64,-2.37,;-13.43,-3.18,;-15.78,-.09,;-17.19,-.68,;-17.34,-2.22,;-18.55,.02,;-18.4,1.67,;-17.08,2.44,;-17.08,3.97,;-15.61,4.72,;-14.35,3.82,;-13,4.55,;-14.45,2.26,;-15.78,1.52,;-19.9,-.62,;-21.14,.17,;-21.14,1.73,;-22.53,-.62,;-22.53,-2.12,;-21.33,-2.88,;-23.81,.17,;-25.13,-.41,;-26.27,.45,;-25.2,-1.9,;-9.26,-1,;-9.3,-2.5,;-7.94,-.34,;-6.59,-1.15,;-6.59,-2.71,;-7.94,-3.46,;-7.94,-5,;-9.3,-5.6,;-6.63,-5.81,;-5.24,-.34,;-5.2,1.15,;-3.86,-1.11,;-2.57,-.23,;-2.57,1.26,;-1.19,2.05,;.1,1.47,;1.29,2.58,;.52,3.87,;-.97,3.65,;-1.19,-1,;-1.19,-2.5,;.1,-.3,;1.55,-1.15,;1.55,-2.65,;2.79,-3.4,;2.79,-4.89,;4.22,-5.7,;5.56,-4.89,;5.56,-3.4,;4.22,-2.65,;2.79,-.34,;2.79,1.2,;4.22,-1,;5.33,-.09,;5.29,1.47,;6.63,2.26,;6.53,3.82,;7.81,4.72,;7.81,6.13,;9.13,7.03,;6.46,6.92,;6.85,-.73,;7,-2.37,;8,.13,;9.35,-.41,;9.69,-2.07,;10.95,-2.71,;12.29,-1.9,;13.51,-2.99,;12.83,-4.36,;13.43,-5.81,;12.55,-7.03,;10.95,-6.77,;10.33,-5.43,;11.29,-4.21,;10.69,.41,;10.48,2.16,;12.04,-.41,;13.38,.3,;14.58,-.51,;14.47,-2.03,;15.99,.17,;17.25,-.68,;17.25,-2.26,;18.49,-3.08,;18.45,-4.57,;19.73,-5.55,;19.69,-7.03,;18.66,.02,;18.77,1.52,;20.01,-.68,;20.09,-2.22,;21.72,-2.54,;22.29,-1.11,;21.5,-.09,;21.78,1.47,;20.65,2.84,;23.32,1.64,;23.83,2.97,;23,4.19,;23.6,5.58,;21.5,4.04,;25.41,3.12,;26.03,4.44,;26.27,1.94,)| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 4 receptor (hMC4R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM82411

(CAS_75921-69-6 | NDP-MSH)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:66.68,55.56,45.45,111.116,4.4,14.23,wD:77.79,95.99,107.113,36.36,8.10,26.29,(-7.66,4.36,;-8.98,3.65,;-9.15,2.12,;-10.5,1.37,;-10.5,-.23,;-11.93,-.94,;-13.21,-.15,;-13.17,1.41,;-14.6,-.83,;-14.64,-2.37,;-13.43,-3.18,;-15.78,-.09,;-17.19,-.68,;-17.34,-2.22,;-18.55,.02,;-18.4,1.67,;-17.08,2.44,;-17.08,3.97,;-15.61,4.72,;-14.35,3.82,;-13,4.55,;-14.45,2.26,;-15.78,1.52,;-19.9,-.62,;-21.14,.17,;-21.14,1.73,;-22.53,-.62,;-22.53,-2.12,;-21.33,-2.88,;-23.81,.17,;-25.13,-.41,;-26.27,.45,;-25.2,-1.9,;-9.26,-1,;-9.3,-2.5,;-7.94,-.34,;-6.59,-1.15,;-6.59,-2.71,;-7.94,-3.46,;-7.94,-5,;-9.3,-5.6,;-6.63,-5.81,;-5.24,-.34,;-5.2,1.15,;-3.86,-1.11,;-2.57,-.23,;-2.57,1.26,;-1.19,2.05,;.1,1.47,;1.29,2.58,;.52,3.87,;-.97,3.65,;-1.19,-1,;-1.19,-2.5,;.1,-.3,;1.55,-1.15,;1.55,-2.65,;2.79,-3.4,;2.79,-4.89,;4.22,-5.7,;5.56,-4.89,;5.56,-3.4,;4.22,-2.65,;2.79,-.34,;2.79,1.2,;4.22,-1,;5.33,-.09,;5.29,1.47,;6.63,2.26,;6.53,3.82,;7.81,4.72,;7.81,6.13,;9.13,7.03,;6.46,6.92,;6.85,-.73,;7,-2.37,;8,.13,;9.35,-.41,;9.69,-2.07,;10.95,-2.71,;12.29,-1.9,;13.51,-2.99,;12.83,-4.36,;13.43,-5.81,;12.55,-7.03,;10.95,-6.77,;10.33,-5.43,;11.29,-4.21,;10.69,.41,;10.48,2.16,;12.04,-.41,;13.38,.3,;14.58,-.51,;14.47,-2.03,;15.99,.17,;17.25,-.68,;17.25,-2.26,;18.49,-3.08,;18.45,-4.57,;19.73,-5.55,;19.69,-7.03,;18.66,.02,;18.77,1.52,;20.01,-.68,;20.09,-2.22,;21.72,-2.54,;22.29,-1.11,;21.5,-.09,;21.78,1.47,;20.65,2.84,;23.32,1.64,;23.83,2.97,;23,4.19,;23.6,5.58,;21.5,4.04,;25.41,3.12,;26.03,4.44,;26.27,1.94,)| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 3 receptor (hMC3R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50035832
(Ac-Trp-L-3-MPt(Et)-Asp-Phe-NH2 | CHEMBL35471)Show SMILES CCS[C@@H]1CCN([C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O Show InChI InChI=1S/C33H40N6O7S/c1-3-47-27-13-14-39(33(46)26(36-19(2)40)16-21-18-35-23-12-8-7-11-22(21)23)29(27)32(45)38-25(17-28(41)42)31(44)37-24(30(34)43)15-20-9-5-4-6-10-20/h4-12,18,24-27,29,35H,3,13-17H2,1-2H3,(H2,34,43)(H,36,40)(H,37,44)(H,38,45)(H,41,42)/t24-,25-,26-,27+,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells |
J Med Chem 38: 137-49 (1995)
BindingDB Entry DOI: 10.7270/Q2QN65TS |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50058160
((3R,6S,9R,12S,15R,18R,26R)-18-Acetylamino-12-benzy...)Show SMILES C[C@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H](CC(=O)NCCCC[C@@H](NC1=O)C(N)=O)NC(C)=O Show InChI InChI=1S/C47H63N15O9/c1-26-41(66)58-33(40(48)65)15-8-9-17-52-39(64)22-38(57-27(2)63)46(71)62-37(21-30-24-51-25-55-30)45(70)60-35(19-28-11-4-3-5-12-28)44(69)59-34(16-10-18-53-47(49)50)42(67)61-36(43(68)56-26)20-29-23-54-32-14-7-6-13-31(29)32/h3-7,11-14,23-26,33-38,54H,8-10,15-22H2,1-2H3,(H2,48,65)(H,51,55)(H,52,64)(H,56,68)(H,57,63)(H,58,66)(H,59,69)(H,60,70)(H,61,67)(H,62,71)(H4,49,50,53)/t26-,33-,34-,35+,36+,37-,38-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 1 receptor (hMC1R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50058160

((3R,6S,9R,12S,15R,18R,26R)-18-Acetylamino-12-benzy...)Show SMILES C[C@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H](CC(=O)NCCCC[C@@H](NC1=O)C(N)=O)NC(C)=O Show InChI InChI=1S/C47H63N15O9/c1-26-41(66)58-33(40(48)65)15-8-9-17-52-39(64)22-38(57-27(2)63)46(71)62-37(21-30-24-51-25-55-30)45(70)60-35(19-28-11-4-3-5-12-28)44(69)59-34(16-10-18-53-47(49)50)42(67)61-36(43(68)56-26)20-29-23-54-32-14-7-6-13-31(29)32/h3-7,11-14,23-26,33-38,54H,8-10,15-22H2,1-2H3,(H2,48,65)(H,51,55)(H,52,64)(H,56,68)(H,57,63)(H,58,66)(H,59,69)(H,60,70)(H,61,67)(H,62,71)(H4,49,50,53)/t26-,33-,34-,35+,36+,37-,38-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 4 receptor (hMC4R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50001468

((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C37H52N8O10/c1-19(2)30(36(54)40-18-28(39)47)45-37(55)31(20(3)4)44-35(53)27(17-29(48)49)43-34(52)26(16-22-9-7-6-8-10-22)42-32(50)21(5)41-33(51)25(38)15-23-11-13-24(46)14-12-23/h6-14,19-21,25-27,30-31,46H,15-18,38H2,1-5H3,(H2,39,47)(H,40,54)(H,41,51)(H,42,50)(H,43,52)(H,44,53)(H,45,55)(H,48,49)/t21-,25+,26+,27+,30+,31+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Guinea pig ileum smooth muscle |
Bioorg Med Chem Lett 2: 547-552 (1992)
Article DOI: 10.1016/S0960-894X(01)81195-1
BindingDB Entry DOI: 10.7270/Q23R0STD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50280456

(CHEMBL153466 | {(4S,7R,10S,13R)-13-[(S)-2-Amino-3-...)Show SMILES CC(C)[C@H](NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CC(O)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C35H46N8O10S2/c1-18(2)29(35(53)38-15-27(37)45)43-34(52)26-17-55-54-16-25(41-30(48)22(36)12-20-8-10-21(44)11-9-20)33(51)39-23(13-19-6-4-3-5-7-19)31(49)40-24(14-28(46)47)32(50)42-26/h3-11,18,22-26,29,44H,12-17,36H2,1-2H3,(H2,37,45)(H,38,53)(H,39,51)(H,40,49)(H,41,48)(H,42,50)(H,43,52)(H,46,47)/t22-,23-,24+,25-,26+,29-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Guinea pig ileum smooth muscle |
Bioorg Med Chem Lett 2: 547-552 (1992)
Article DOI: 10.1016/S0960-894X(01)81195-1
BindingDB Entry DOI: 10.7270/Q23R0STD |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50035825
(Ac-Trp-L-3-MPt(Pr)-Asp-Phe-NH2 | CHEMBL34554)Show SMILES CCCS[C@H]1CCN([C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O Show InChI InChI=1S/C34H42N6O7S/c1-3-15-48-28-13-14-40(34(47)27(37-20(2)41)17-22-19-36-24-12-8-7-11-23(22)24)30(28)33(46)39-26(18-29(42)43)32(45)38-25(31(35)44)16-21-9-5-4-6-10-21/h4-12,19,25-28,30,36H,3,13-18H2,1-2H3,(H2,35,44)(H,37,41)(H,38,45)(H,39,46)(H,42,43)/t25-,26-,27-,28-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells |
J Med Chem 38: 137-49 (1995)
BindingDB Entry DOI: 10.7270/Q2QN65TS |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50001683
(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor delta 1 was determined in guinea pig wholebrain using [3H][4''-Cl-Phe4]-DPDPE as radioligand |
J Med Chem 39: 4120-4 (1996)
Article DOI: 10.1021/jm960078j
BindingDB Entry DOI: 10.7270/Q2P55P5Z |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50280456

(CHEMBL153466 | {(4S,7R,10S,13R)-13-[(S)-2-Amino-3-...)Show SMILES CC(C)[C@H](NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CC(O)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C35H46N8O10S2/c1-18(2)29(35(53)38-15-27(37)45)43-34(52)26-17-55-54-16-25(41-30(48)22(36)12-20-8-10-21(44)11-9-20)33(51)39-23(13-19-6-4-3-5-7-19)31(49)40-24(14-28(46)47)32(50)42-26/h3-11,18,22-26,29,44H,12-17,36H2,1-2H3,(H2,37,45)(H,38,53)(H,39,51)(H,40,49)(H,41,48)(H,42,50)(H,43,52)(H,46,47)/t22-,23-,24+,25-,26+,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 with [3H]-CTOP |
Bioorg Med Chem Lett 2: 547-552 (1992)
Article DOI: 10.1016/S0960-894X(01)81195-1
BindingDB Entry DOI: 10.7270/Q23R0STD |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50029747
((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 1 receptor (hMC1R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 3 receptor (hMC3R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50058158

((3S,6S,9S,12S,15S,18S,26S)-18-((S)-2-Acetylamino-h...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O Show InChI InChI=1S/C53H74N16O10/c1-4-5-17-38(63-31(3)70)47(74)69-43-26-44(71)58-21-12-11-19-37(45(54)72)64-46(73)30(2)62-49(76)41(24-33-27-60-36-18-10-9-16-35(33)36)67-48(75)39(20-13-22-59-53(55)56)65-50(77)40(23-32-14-7-6-8-15-32)66-51(78)42(68-52(43)79)25-34-28-57-29-61-34/h6-10,14-16,18,27-30,37-43,60H,4-5,11-13,17,19-26H2,1-3H3,(H2,54,72)(H,57,61)(H,58,71)(H,62,76)(H,63,70)(H,64,73)(H,65,77)(H,66,78)(H,67,75)(H,68,79)(H,69,74)(H4,55,56,59)/t30-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 1 receptor (hMC1R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50058158

((3S,6S,9S,12S,15S,18S,26S)-18-((S)-2-Acetylamino-h...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O Show InChI InChI=1S/C53H74N16O10/c1-4-5-17-38(63-31(3)70)47(74)69-43-26-44(71)58-21-12-11-19-37(45(54)72)64-46(73)30(2)62-49(76)41(24-33-27-60-36-18-10-9-16-35(33)36)67-48(75)39(20-13-22-59-53(55)56)65-50(77)40(23-32-14-7-6-8-15-32)66-51(78)42(68-52(43)79)25-34-28-57-29-61-34/h6-10,14-16,18,27-30,37-43,60H,4-5,11-13,17,19-26H2,1-3H3,(H2,54,72)(H,57,61)(H,58,71)(H,62,76)(H,63,70)(H,64,73)(H,65,77)(H,66,78)(H,67,75)(H,68,79)(H,69,74)(H4,55,56,59)/t30-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 1 receptor (hMC1R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50454555

(CHEMBL2115441)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O |wU:30.30,66.69,4.4,44.46,25.25,wD:12.11,21.80,55.57,(-3.6,-3.27,;-2.8,-4.62,;-3.51,-6.01,;-2.66,-7.31,;-3.24,-8.75,;-4.77,-8.95,;-5.17,-10.44,;-6.68,-10.84,;-4.09,-11.55,;-2.3,-9.96,;-2.87,-11.38,;-.49,-9.48,;.9,-10.13,;.41,-11.59,;.29,-13.13,;-1.26,-13.19,;.53,-14.65,;1.14,-16.07,;2.06,-17.29,;3.26,-18.26,;4.65,-18.92,;6.15,-19.23,;7.69,-19.18,;9.17,-18.75,;9.77,-20.16,;10.5,-17.98,;11.43,-19.2,;11.62,-16.91,;12.44,-15.6,;13.83,-16.26,;12.93,-14.14,;14.44,-14.44,;14.92,-15.92,;16.39,-16.38,;16.4,-17.92,;14.94,-18.4,;14.33,-19.81,;12.8,-19.99,;11.88,-18.75,;12.49,-17.34,;14.02,-17.17,;13.05,-12.61,;12.8,-11.09,;14.28,-10.67,;12.2,-9.68,;13.53,-8.91,;14.87,-9.68,;16.19,-8.91,;17.53,-9.68,;18.87,-8.91,;20.19,-9.68,;18.87,-7.37,;11.27,-8.44,;10.09,-7.47,;10.9,-6.17,;8.69,-6.81,;9.17,-5.35,;10.69,-5.05,;11.17,-3.59,;12.68,-3.28,;13.71,-4.44,;13.22,-5.89,;11.7,-6.19,;7.18,-6.51,;5.64,-6.55,;5.92,-5.52,;4.17,-6.99,;3.56,-5.58,;4.48,-4.34,;6.03,-4.38,;6.52,-2.9,;5.3,-1.99,;4.03,-2.88,;2.83,-7.77,;1.72,-8.83,;.53,-7.85,;6.03,-20.77,;7.29,-21.65,;4.64,-21.43,)| Show InChI InChI=1S/C53H74N16O10/c1-4-5-17-38(63-31(3)70)47(74)69-43-26-44(71)58-21-12-11-19-37(45(54)72)64-46(73)30(2)62-49(76)41(24-33-27-60-36-18-10-9-16-35(33)36)67-48(75)39(20-13-22-59-53(55)56)65-50(77)40(23-32-14-7-6-8-15-32)66-51(78)42(68-52(43)79)25-34-28-57-29-61-34/h6-10,14-16,18,27-30,37-43,60H,4-5,11-13,17,19-26H2,1-3H3,(H2,54,72)(H,57,61)(H,58,71)(H,62,76)(H,63,70)(H,64,73)(H,65,77)(H,66,78)(H,67,75)(H,68,79)(H,69,74)(H4,55,56,59)/t30-,37-,38-,39-,40+,41-,42-,43-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 5 receptor (hMC5R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50454555

(CHEMBL2115441)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O |wU:30.30,66.69,4.4,44.46,25.25,wD:12.11,21.80,55.57,(-3.6,-3.27,;-2.8,-4.62,;-3.51,-6.01,;-2.66,-7.31,;-3.24,-8.75,;-4.77,-8.95,;-5.17,-10.44,;-6.68,-10.84,;-4.09,-11.55,;-2.3,-9.96,;-2.87,-11.38,;-.49,-9.48,;.9,-10.13,;.41,-11.59,;.29,-13.13,;-1.26,-13.19,;.53,-14.65,;1.14,-16.07,;2.06,-17.29,;3.26,-18.26,;4.65,-18.92,;6.15,-19.23,;7.69,-19.18,;9.17,-18.75,;9.77,-20.16,;10.5,-17.98,;11.43,-19.2,;11.62,-16.91,;12.44,-15.6,;13.83,-16.26,;12.93,-14.14,;14.44,-14.44,;14.92,-15.92,;16.39,-16.38,;16.4,-17.92,;14.94,-18.4,;14.33,-19.81,;12.8,-19.99,;11.88,-18.75,;12.49,-17.34,;14.02,-17.17,;13.05,-12.61,;12.8,-11.09,;14.28,-10.67,;12.2,-9.68,;13.53,-8.91,;14.87,-9.68,;16.19,-8.91,;17.53,-9.68,;18.87,-8.91,;20.19,-9.68,;18.87,-7.37,;11.27,-8.44,;10.09,-7.47,;10.9,-6.17,;8.69,-6.81,;9.17,-5.35,;10.69,-5.05,;11.17,-3.59,;12.68,-3.28,;13.71,-4.44,;13.22,-5.89,;11.7,-6.19,;7.18,-6.51,;5.64,-6.55,;5.92,-5.52,;4.17,-6.99,;3.56,-5.58,;4.48,-4.34,;6.03,-4.38,;6.52,-2.9,;5.3,-1.99,;4.03,-2.88,;2.83,-7.77,;1.72,-8.83,;.53,-7.85,;6.03,-20.77,;7.29,-21.65,;4.64,-21.43,)| Show InChI InChI=1S/C53H74N16O10/c1-4-5-17-38(63-31(3)70)47(74)69-43-26-44(71)58-21-12-11-19-37(45(54)72)64-46(73)30(2)62-49(76)41(24-33-27-60-36-18-10-9-16-35(33)36)67-48(75)39(20-13-22-59-53(55)56)65-50(77)40(23-32-14-7-6-8-15-32)66-51(78)42(68-52(43)79)25-34-28-57-29-61-34/h6-10,14-16,18,27-30,37-43,60H,4-5,11-13,17,19-26H2,1-3H3,(H2,54,72)(H,57,61)(H,58,71)(H,62,76)(H,63,70)(H,64,73)(H,65,77)(H,66,78)(H,67,75)(H,68,79)(H,69,74)(H4,55,56,59)/t30-,37-,38-,39-,40+,41-,42-,43-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 3 receptor (hMC3R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21146

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-[(3S)-3-...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C55H66N10O13/c1-3-4-19-45(54(77)64-43(29-48(70)71)53(76)61-40(49(57)72)24-32-13-7-5-8-14-32)65(2)55(78)44(27-35-30-58-39-18-12-11-17-37(35)39)60-46(67)31-59-51(74)41(25-33-15-9-6-10-16-33)63-52(75)42(26-34-20-22-36(66)23-21-34)62-50(73)38(56)28-47(68)69/h5-18,20-23,30,38,40-45,58,66H,3-4,19,24-29,31,56H2,1-2H3,(H2,57,72)(H,59,74)(H,60,67)(H,61,76)(H,62,73)(H,63,75)(H,64,77)(H,68,69)(H,70,71)/t38-,40-,41+,42-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor delta 1 was determined in guinea pig wholebrain using [3H][4''-Cl-Phe4]-DPDPE as radioligand |
J Med Chem 39: 4120-4 (1996)
Article DOI: 10.1021/jm960078j
BindingDB Entry DOI: 10.7270/Q2P55P5Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50035829
(Ac-Trp-L-3-MPt(Et)-Asp-Phe-NH2 | CHEMBL34853)Show SMILES CCS[C@H]1CCN([C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O Show InChI InChI=1S/C33H40N6O7S/c1-3-47-27-13-14-39(33(46)26(36-19(2)40)16-21-18-35-23-12-8-7-11-22(21)23)29(27)32(45)38-25(17-28(41)42)31(44)37-24(30(34)43)15-20-9-5-4-6-10-20/h4-12,18,24-27,29,35H,3,13-17H2,1-2H3,(H2,34,43)(H,36,40)(H,37,44)(H,38,45)(H,41,42)/t24-,25-,26-,27-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells |
J Med Chem 38: 137-49 (1995)
BindingDB Entry DOI: 10.7270/Q2QN65TS |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50029747

((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 4 receptor (hMC4R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 5 receptor (hMC5R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50035834

(Ac-Trp-L-3-MPt(Me)-Asp-Phe-NH2 | CHEMBL287753)Show SMILES CS[C@@H]1CCN([C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O Show InChI InChI=1S/C32H38N6O7S/c1-18(39)35-25(15-20-17-34-22-11-7-6-10-21(20)22)32(45)38-13-12-26(46-2)28(38)31(44)37-24(16-27(40)41)30(43)36-23(29(33)42)14-19-8-4-3-5-9-19/h3-11,17,23-26,28,34H,12-16H2,1-2H3,(H2,33,42)(H,35,39)(H,36,43)(H,37,44)(H,40,41)/t23-,24-,25-,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells |
J Med Chem 38: 137-49 (1995)
BindingDB Entry DOI: 10.7270/Q2QN65TS |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50029747

((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 3 receptor (hMC3R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50422178

(CHEMBL2112688)Show SMILES CCCC[C@H](N(C)C(=O)[C@@H](NC(=O)CNC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)CC(O)=O)[C@H](C)c1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C56H68N10O13/c1-4-5-20-45(55(78)64-44(29-48(71)72)54(77)61-41(50(58)73)25-33-14-8-6-9-15-33)66(3)56(79)49(32(2)38-30-59-40-19-13-12-18-37(38)40)65-46(68)31-60-52(75)42(26-34-16-10-7-11-17-34)63-53(76)43(27-35-21-23-36(67)24-22-35)62-51(74)39(57)28-47(69)70/h6-19,21-24,30,32,39,41-45,49,59,67H,4-5,20,25-29,31,57H2,1-3H3,(H2,58,73)(H,60,75)(H,61,77)(H,62,74)(H,63,76)(H,64,78)(H,65,68)(H,69,70)(H,71,72)/t32-,39+,41+,42-,43+,44+,45+,49+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor delta 1 was determined in guinea pig wholebrain using [3H][4''-Cl-Phe4]-DPDPE as radioligand |
J Med Chem 39: 4120-4 (1996)
Article DOI: 10.1021/jm960078j
BindingDB Entry DOI: 10.7270/Q2P55P5Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50035833

(Ac-Trp-D-3-MPt(Pr)-Asp-Phe-NH2 | CHEMBL37984)Show SMILES CCCS[C@@H]1CCN([C@@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O Show InChI InChI=1S/C34H42N6O7S/c1-3-15-48-28-13-14-40(34(47)27(37-20(2)41)17-22-19-36-24-12-8-7-11-23(22)24)30(28)33(46)39-26(18-29(42)43)32(45)38-25(31(35)44)16-21-9-5-4-6-10-21/h4-12,19,25-28,30,36H,3,13-18H2,1-2H3,(H2,35,44)(H,37,41)(H,38,45)(H,39,46)(H,42,43)/t25-,26-,27-,28+,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells |
J Med Chem 38: 137-49 (1995)
BindingDB Entry DOI: 10.7270/Q2QN65TS |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50058158

((3S,6S,9S,12S,15S,18S,26S)-18-((S)-2-Acetylamino-h...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O Show InChI InChI=1S/C53H74N16O10/c1-4-5-17-38(63-31(3)70)47(74)69-43-26-44(71)58-21-12-11-19-37(45(54)72)64-46(73)30(2)62-49(76)41(24-33-27-60-36-18-10-9-16-35(33)36)67-48(75)39(20-13-22-59-53(55)56)65-50(77)40(23-32-14-7-6-8-15-32)66-51(78)42(68-52(43)79)25-34-28-57-29-61-34/h6-10,14-16,18,27-30,37-43,60H,4-5,11-13,17,19-26H2,1-3H3,(H2,54,72)(H,57,61)(H,58,71)(H,62,76)(H,63,70)(H,64,73)(H,65,77)(H,66,78)(H,67,75)(H,68,79)(H,69,74)(H4,55,56,59)/t30-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 4 receptor (hMC4R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50422175
(CHEMBL2112689)Show SMILES CCCC[C@H](N(C)C(=O)[C@@H](NC(=O)CNC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)CC(O)=O)[C@@H](C)c1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C56H68N10O13/c1-4-5-20-45(55(78)64-44(29-48(71)72)54(77)61-41(50(58)73)25-33-14-8-6-9-15-33)66(3)56(79)49(32(2)38-30-59-40-19-13-12-18-37(38)40)65-46(68)31-60-52(75)42(26-34-16-10-7-11-17-34)63-53(76)43(27-35-21-23-36(67)24-22-35)62-51(74)39(57)28-47(69)70/h6-19,21-24,30,32,39,41-45,49,59,67H,4-5,20,25-29,31,57H2,1-3H3,(H2,58,73)(H,60,75)(H,61,77)(H,62,74)(H,63,76)(H,64,78)(H,65,68)(H,69,70)(H,71,72)/t32-,39-,41-,42+,43-,44-,45-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor was determined in guinea pig cortex using [3H]SNF8702 as radioligand |
J Med Chem 39: 4120-4 (1996)
Article DOI: 10.1021/jm960078j
BindingDB Entry DOI: 10.7270/Q2P55P5Z |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50058161
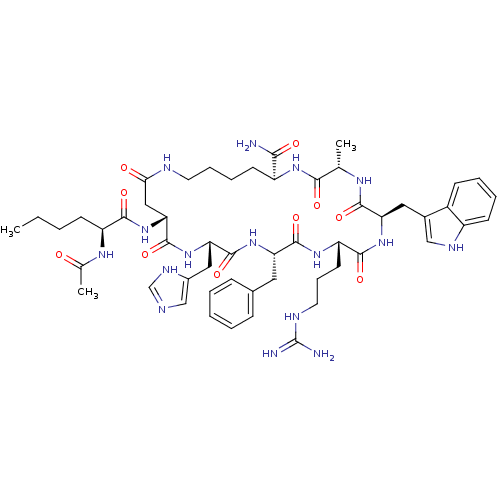
((3S,6R,9S,12S,15S,18R,26S)-18-((S)-2-Acetylamino-h...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O Show InChI InChI=1S/C53H74N16O10/c1-4-5-17-38(63-31(3)70)47(74)69-43-26-44(71)58-21-12-11-19-37(45(54)72)64-46(73)30(2)62-49(76)41(24-33-27-60-36-18-10-9-16-35(33)36)67-48(75)39(20-13-22-59-53(55)56)65-50(77)40(23-32-14-7-6-8-15-32)66-51(78)42(68-52(43)79)25-34-28-57-29-61-34/h6-10,14-16,18,27-30,37-43,60H,4-5,11-13,17,19-26H2,1-3H3,(H2,54,72)(H,57,61)(H,58,71)(H,62,76)(H,63,70)(H,64,73)(H,65,77)(H,66,78)(H,67,75)(H,68,79)(H,69,74)(H4,55,56,59)/t30-,37-,38-,39-,40-,41+,42-,43+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 4 receptor (hMC4R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50058160

((3R,6S,9R,12S,15R,18R,26R)-18-Acetylamino-12-benzy...)Show SMILES C[C@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H](CC(=O)NCCCC[C@@H](NC1=O)C(N)=O)NC(C)=O Show InChI InChI=1S/C47H63N15O9/c1-26-41(66)58-33(40(48)65)15-8-9-17-52-39(64)22-38(57-27(2)63)46(71)62-37(21-30-24-51-25-55-30)45(70)60-35(19-28-11-4-3-5-12-28)44(69)59-34(16-10-18-53-47(49)50)42(67)61-36(43(68)56-26)20-29-23-54-32-14-7-6-13-31(29)32/h3-7,11-14,23-26,33-38,54H,8-10,15-22H2,1-2H3,(H2,48,65)(H,51,55)(H,52,64)(H,56,68)(H,57,63)(H,58,66)(H,59,69)(H,60,70)(H,61,67)(H,62,71)(H4,49,50,53)/t26-,33-,34-,35+,36+,37-,38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 3 receptor (hMC3R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50422175

(CHEMBL2112689)Show SMILES CCCC[C@H](N(C)C(=O)[C@@H](NC(=O)CNC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)CC(O)=O)[C@@H](C)c1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C56H68N10O13/c1-4-5-20-45(55(78)64-44(29-48(71)72)54(77)61-41(50(58)73)25-33-14-8-6-9-15-33)66(3)56(79)49(32(2)38-30-59-40-19-13-12-18-37(38)40)65-46(68)31-60-52(75)42(26-34-16-10-7-11-17-34)63-53(76)43(27-35-21-23-36(67)24-22-35)62-51(74)39(57)28-47(69)70/h6-19,21-24,30,32,39,41-45,49,59,67H,4-5,20,25-29,31,57H2,1-3H3,(H2,58,73)(H,60,75)(H,61,77)(H,62,74)(H,63,76)(H,64,78)(H,65,68)(H,69,70)(H,71,72)/t32-,39-,41-,42+,43-,44-,45-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor delta 1 was determined in guinea pig wholebrain using [3H][4''-Cl-Phe4]-DPDPE as radioligand |
J Med Chem 39: 4120-4 (1996)
Article DOI: 10.1021/jm960078j
BindingDB Entry DOI: 10.7270/Q2P55P5Z |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50058158

((3S,6S,9S,12S,15S,18S,26S)-18-((S)-2-Acetylamino-h...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O Show InChI InChI=1S/C53H74N16O10/c1-4-5-17-38(63-31(3)70)47(74)69-43-26-44(71)58-21-12-11-19-37(45(54)72)64-46(73)30(2)62-49(76)41(24-33-27-60-36-18-10-9-16-35(33)36)67-48(75)39(20-13-22-59-53(55)56)65-50(77)40(23-32-14-7-6-8-15-32)66-51(78)42(68-52(43)79)25-34-28-57-29-61-34/h6-10,14-16,18,27-30,37-43,60H,4-5,11-13,17,19-26H2,1-3H3,(H2,54,72)(H,57,61)(H,58,71)(H,62,76)(H,63,70)(H,64,73)(H,65,77)(H,66,78)(H,67,75)(H,68,79)(H,69,74)(H4,55,56,59)/t30-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 5 receptor (hMC5R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50058161

((3S,6R,9S,12S,15S,18R,26S)-18-((S)-2-Acetylamino-h...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O Show InChI InChI=1S/C53H74N16O10/c1-4-5-17-38(63-31(3)70)47(74)69-43-26-44(71)58-21-12-11-19-37(45(54)72)64-46(73)30(2)62-49(76)41(24-33-27-60-36-18-10-9-16-35(33)36)67-48(75)39(20-13-22-59-53(55)56)65-50(77)40(23-32-14-7-6-8-15-32)66-51(78)42(68-52(43)79)25-34-28-57-29-61-34/h6-10,14-16,18,27-30,37-43,60H,4-5,11-13,17,19-26H2,1-3H3,(H2,54,72)(H,57,61)(H,58,71)(H,62,76)(H,63,70)(H,64,73)(H,65,77)(H,66,78)(H,67,75)(H,68,79)(H,69,74)(H4,55,56,59)/t30-,37-,38-,39-,40-,41+,42-,43+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 264 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 1 receptor (hMC1R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50035822
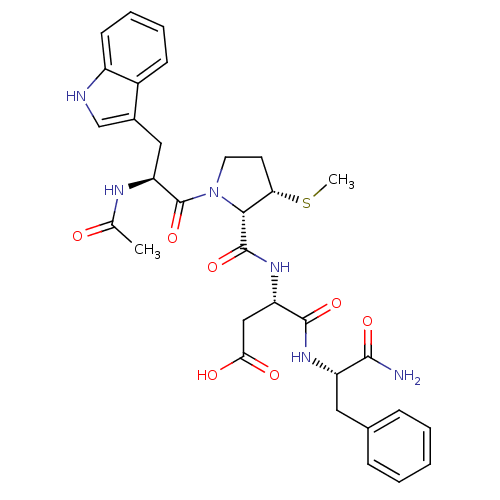
(Ac-Trp-L-3-MPc(Me)-Asp-Phe-NH2 | CHEMBL37983)Show SMILES CS[C@H]1CCN([C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O Show InChI InChI=1S/C32H38N6O7S/c1-18(39)35-25(15-20-17-34-22-11-7-6-10-21(20)22)32(45)38-13-12-26(46-2)28(38)31(44)37-24(16-27(40)41)30(43)36-23(29(33)42)14-19-8-4-3-5-9-19/h3-11,17,23-26,28,34H,12-16H2,1-2H3,(H2,33,42)(H,35,39)(H,36,43)(H,37,44)(H,40,41)/t23-,24-,25-,26-,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells |
J Med Chem 38: 137-49 (1995)
BindingDB Entry DOI: 10.7270/Q2QN65TS |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50237601
(CHEMBL411941 | CycloRGDfV | [(2S,5R,8S,11S)-5-Benz...)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C26H38N8O7/c1-14(2)21-25(41)32-16(9-6-10-29-26(27)28)22(38)30-13-19(35)31-18(12-20(36)37)23(39)33-17(24(40)34-21)11-15-7-4-3-5-8-15/h3-5,7-8,14,16-18,21H,6,9-13H2,1-2H3,(H,30,38)(H,31,35)(H,32,41)(H,33,39)(H,34,40)(H,36,37)(H4,27,28,29)/t16-,17+,18-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [125I]echistatin from human integrin alpha5beta3 expressed in human A549 cells by binding assay |
Bioorg Med Chem Lett 21: 2116-20 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.133
BindingDB Entry DOI: 10.7270/Q23779ZW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50029747

((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 557 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 5 receptor (hMC5R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50035823
(Ac-Trp-D-Pro-Asp-Phe-NH2 | Ac-Trp-Pro-Asp-Phe-NH2 ...)Show SMILES CC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C31H36N6O7/c1-18(38)34-25(15-20-17-33-22-11-6-5-10-21(20)22)31(44)37-13-7-12-26(37)30(43)36-24(16-27(39)40)29(42)35-23(28(32)41)14-19-8-3-2-4-9-19/h2-6,8-11,17,23-26,33H,7,12-16H2,1H3,(H2,32,41)(H,34,38)(H,35,42)(H,36,43)(H,39,40)/t23-,24-,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor by displacement of [125I]-BH-CCK-8 from human jurkat cells |
J Med Chem 38: 137-49 (1995)
BindingDB Entry DOI: 10.7270/Q2QN65TS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































