 Found 89 hits with Last Name = 'nisato' and Initial = 'd'
Found 89 hits with Last Name = 'nisato' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(BOVINE) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282260

(7-Butyl-8-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCC1=NC2(CCCC2)CC(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C26H30N6O/c1-2-3-10-23-27-26(15-6-7-16-26)17-24(33)32(23)18-19-11-13-20(14-12-19)21-8-4-5-9-22(21)25-28-30-31-29-25/h4-5,8-9,11-14H,2-3,6-7,10,15-18H2,1H3,(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50042235
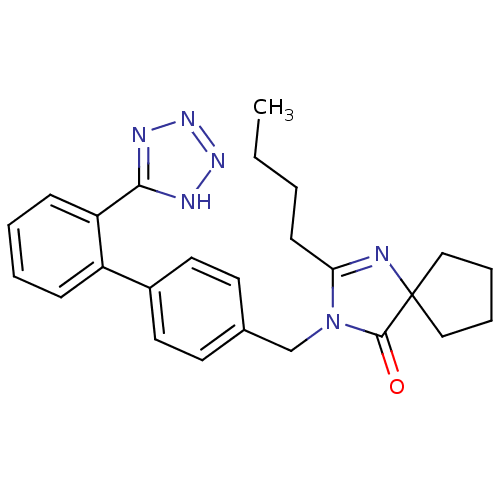
(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282261
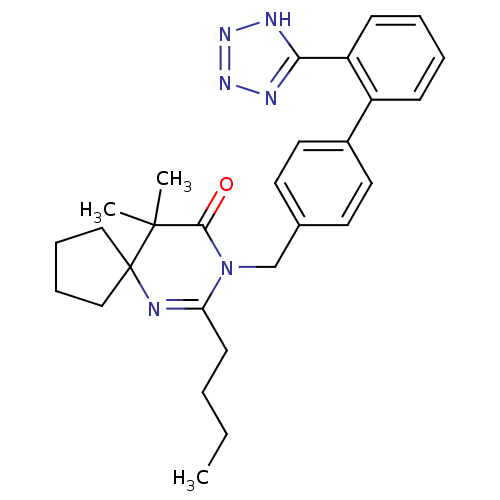
(7-Butyl-10,10-dimethyl-8-[2'-(2H-tetrazol-5-yl)-bi...)Show SMILES CCCCC1=NC2(CCCC2)C(C)(C)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C28H34N6O/c1-4-5-12-24-29-28(17-8-9-18-28)27(2,3)26(35)34(24)19-20-13-15-21(16-14-20)22-10-6-7-11-23(22)25-30-32-33-31-25/h6-7,10-11,13-16H,4-5,8-9,12,17-19H2,1-3H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282270

(4'-(2-Butyl-4-cyclopentyl-4-methyl-5-oxo-4,5-dihyd...)Show SMILES CCCCC1=NC(C)(C2CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C27H32N2O3/c1-3-4-13-24-28-27(2,21-9-5-6-10-21)26(32)29(24)18-19-14-16-20(17-15-19)22-11-7-8-12-23(22)25(30)31/h7-8,11-12,14-17,21H,3-6,9-10,13,18H2,1-2H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282264
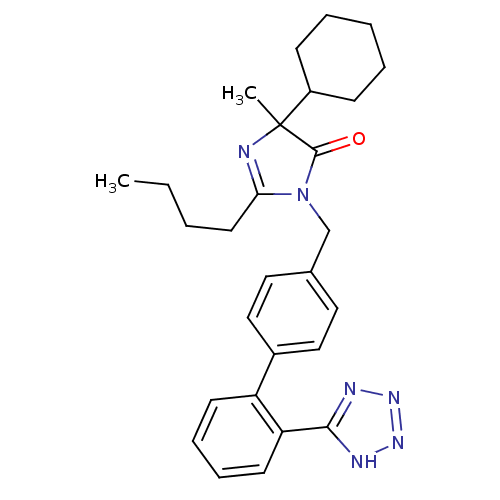
(2-Butyl-5-cyclohexyl-5-methyl-3-[2'-(1H-tetrazol-5...)Show SMILES CCCCC1=NC(C)(C2CCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C28H34N6O/c1-3-4-14-25-29-28(2,22-10-6-5-7-11-22)27(35)34(25)19-20-15-17-21(18-16-20)23-12-8-9-13-24(23)26-30-32-33-31-26/h8-9,12-13,15-18,22H,3-7,10-11,14,19H2,1-2H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282264

(2-Butyl-5-cyclohexyl-5-methyl-3-[2'-(1H-tetrazol-5...)Show SMILES CCCCC1=NC(C)(C2CCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C28H34N6O/c1-3-4-14-25-29-28(2,22-10-6-5-7-11-22)27(35)34(25)19-20-15-17-21(18-16-20)23-12-8-9-13-24(23)26-30-32-33-31-26/h8-9,12-13,15-18,22H,3-7,10-11,14,19H2,1-2H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282267
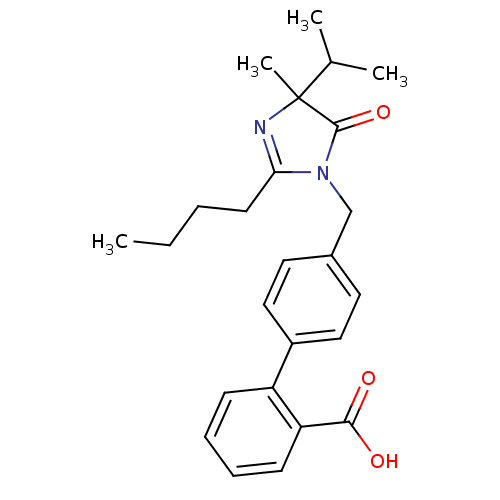
(4'-(2-Butyl-4-isopropyl-4-methyl-5-oxo-4,5-dihydro...)Show SMILES CCCCC1=NC(C)(C(C)C)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C25H30N2O3/c1-5-6-11-22-26-25(4,17(2)3)24(30)27(22)16-18-12-14-19(15-13-18)20-9-7-8-10-21(20)23(28)29/h7-10,12-15,17H,5-6,11,16H2,1-4H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282263
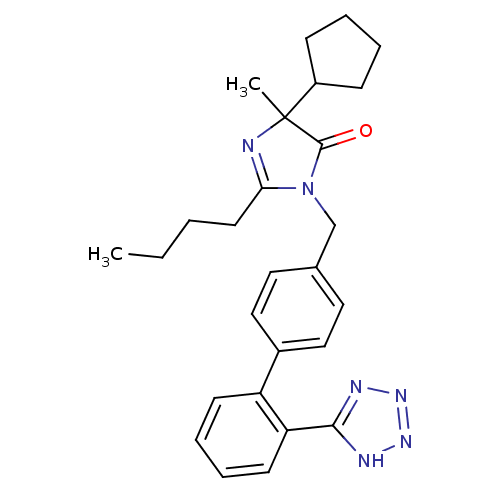
(2-Butyl-5-cyclopentyl-5-methyl-3-[2'-(2H-tetrazol-...)Show SMILES CCCCC1=NC(C)(C2CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C27H32N6O/c1-3-4-13-24-28-27(2,21-9-5-6-10-21)26(34)33(24)18-19-14-16-20(17-15-19)22-11-7-8-12-23(22)25-29-31-32-30-25/h7-8,11-12,14-17,21H,3-6,9-10,13,18H2,1-2H3,(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282262
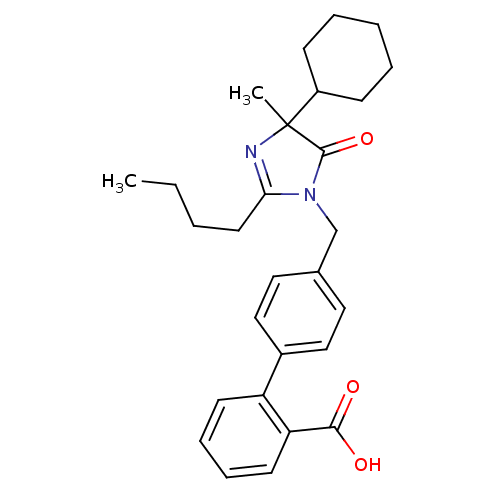
(4'-(2-Butyl-4-cyclohexyl-4-methyl-5-oxo-4,5-dihydr...)Show SMILES CCCCC1=NC(C)(C2CCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C28H34N2O3/c1-3-4-14-25-29-28(2,22-10-6-5-7-11-22)27(33)30(25)19-20-15-17-21(18-16-20)23-12-8-9-13-24(23)26(31)32/h8-9,12-13,15-18,22H,3-7,10-11,14,19H2,1-2H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282268
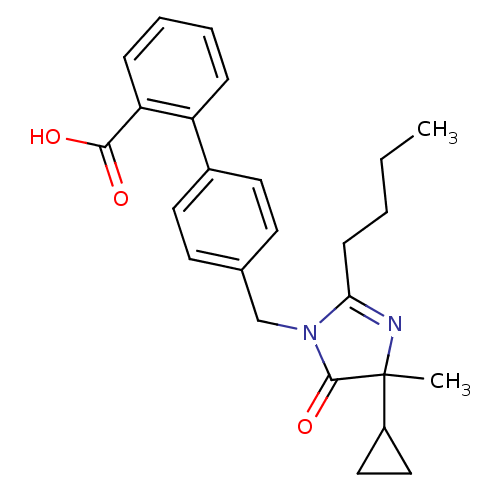
(4'-(2-Butyl-4-cyclopropyl-4-methyl-5-oxo-4,5-dihyd...)Show SMILES CCCCC1=NC(C)(C2CC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C25H28N2O3/c1-3-4-9-22-26-25(2,19-14-15-19)24(30)27(22)16-17-10-12-18(13-11-17)20-7-5-6-8-21(20)23(28)29/h5-8,10-13,19H,3-4,9,14-16H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282265
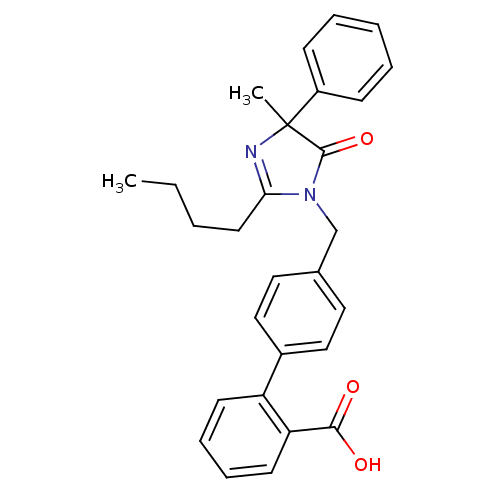
(4'-(2-Butyl-4-methyl-5-oxo-4-phenyl-4,5-dihydro-im...)Show SMILES CCCCC1=NC(C)(C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O)c1ccccc1 |t:4| Show InChI InChI=1S/C28H28N2O3/c1-3-4-14-25-29-28(2,22-10-6-5-7-11-22)27(33)30(25)19-20-15-17-21(18-16-20)23-12-8-9-13-24(23)26(31)32/h5-13,15-18H,3-4,14,19H2,1-2H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025903
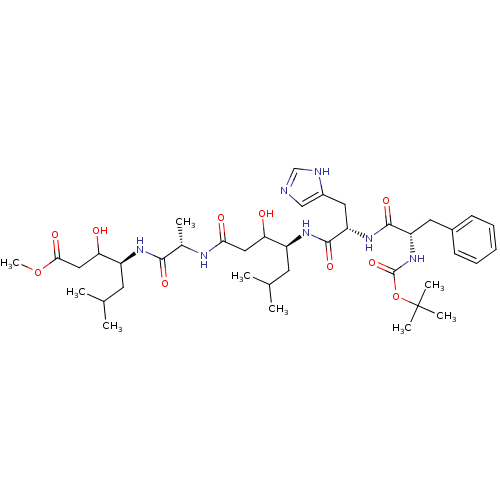
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C40H63N7O10/c1-23(2)15-28(32(48)19-34(50)43-25(5)36(52)44-29(16-24(3)4)33(49)20-35(51)56-9)45-38(54)31(18-27-21-41-22-42-27)46-37(53)30(17-26-13-11-10-12-14-26)47-39(55)57-40(6,7)8/h10-14,21-25,28-33,48-49H,15-20H2,1-9H3,(H,41,42)(H,43,50)(H,44,52)(H,45,54)(H,46,53)(H,47,55)/t25-,28-,29-,30-,31-,32?,33?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024167
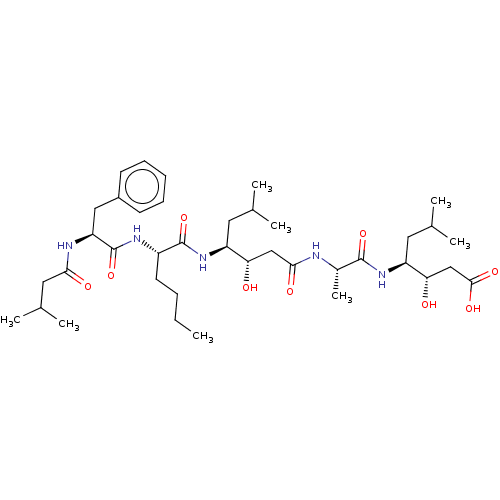
(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O9/c1-9-10-16-28(42-39(53)31(41-34(47)19-25(6)7)20-27-14-12-11-13-15-27)38(52)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(51)43-30(18-24(4)5)33(46)22-36(49)50/h11-15,23-26,28-33,45-46H,9-10,16-22H2,1-8H3,(H,40,48)(H,41,47)(H,42,53)(H,43,51)(H,44,52)(H,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024187
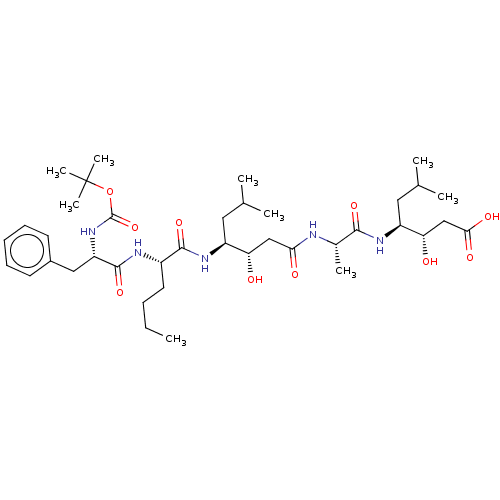
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O10/c1-10-11-17-27(41-37(52)30(20-26-15-13-12-14-16-26)44-38(53)54-39(7,8)9)36(51)43-28(18-23(2)3)31(45)21-33(47)40-25(6)35(50)42-29(19-24(4)5)32(46)22-34(48)49/h12-16,23-25,27-32,45-46H,10-11,17-22H2,1-9H3,(H,40,47)(H,41,52)(H,42,50)(H,43,51)(H,44,53)(H,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50042244
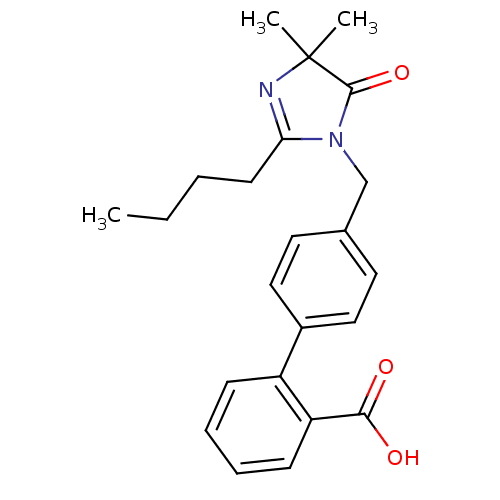
(4'-(2-Butyl-4,4-dimethyl-5-oxo-4,5-dihydro-imidazo...)Show SMILES CCCCC1=NC(C)(C)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C23H26N2O3/c1-4-5-10-20-24-23(2,3)22(28)25(20)15-16-11-13-17(14-12-16)18-8-6-7-9-19(18)21(26)27/h6-9,11-14H,4-5,10,15H2,1-3H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421779

(CHEMBL308300 | CHEMBL3142338)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C1CCCC1 |r| Show InChI InChI=1S/C41H67N5O10/c1-24(2)19-29(32(47)22-34(49)42-26(5)37(51)43-30(20-25(3)4)33(48)23-35(50)55-9)44-39(53)36(28-17-13-14-18-28)46-38(52)31(21-27-15-11-10-12-16-27)45-40(54)56-41(6,7)8/h10-12,15-16,24-26,28-33,36,47-48H,13-14,17-23H2,1-9H3,(H,42,49)(H,43,51)(H,44,53)(H,45,54)(H,46,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421775

(CHEMBL3142326 | CHEMBL73556)Show SMILES CCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C39H65N5O9/c1-10-14-28(42-39(52)31(41-34(47)19-25(6)7)20-27-15-12-11-13-16-27)38(51)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(50)43-30(18-24(4)5)33(46)22-36(49)53-9/h11-13,15-16,23-26,28-33,45-46H,10,14,17-22H2,1-9H3,(H,40,48)(H,41,47)(H,42,52)(H,43,50)(H,44,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50226299

(CHEMBL3142798)Show SMILES CCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C39H65N5O10/c1-11-15-27(41-37(51)30(20-26-16-13-12-14-17-26)44-38(52)54-39(7,8)9)36(50)43-28(18-23(2)3)31(45)21-33(47)40-25(6)35(49)42-29(19-24(4)5)32(46)22-34(48)53-10/h12-14,16-17,23-25,27-32,45-46H,11,15,18-22H2,1-10H3,(H,40,47)(H,41,51)(H,42,49)(H,43,50)(H,44,52)/t25-,27-,28-,29-,30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282269
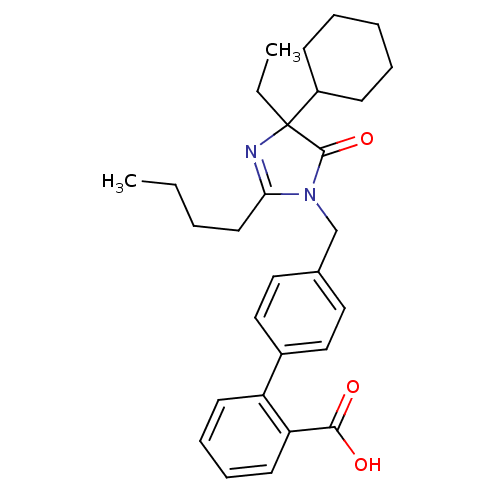
(4'-(2-Butyl-4-cyclohexyl-4-ethyl-5-oxo-4,5-dihydro...)Show SMILES CCCCC1=NC(CC)(C2CCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C29H36N2O3/c1-3-5-15-26-30-29(4-2,23-11-7-6-8-12-23)28(34)31(26)20-21-16-18-22(19-17-21)24-13-9-10-14-25(24)27(32)33/h9-10,13-14,16-19,23H,3-8,11-12,15,20H2,1-2H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282257
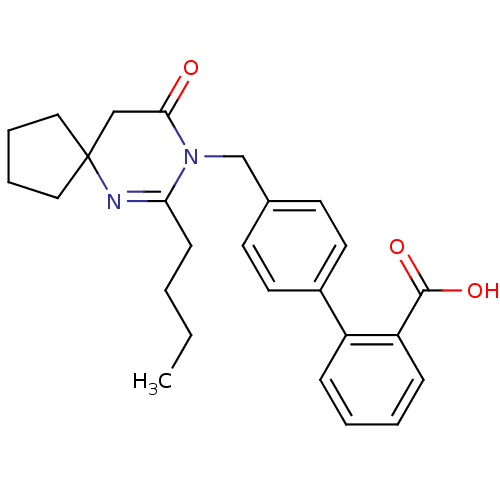
(4'-(7-Butyl-9-oxo-6,8-diaza-spiro[4.5]dec-6-en-8-y...)Show SMILES CCCCC1=NC2(CCCC2)CC(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C26H30N2O3/c1-2-3-10-23-27-26(15-6-7-16-26)17-24(29)28(23)18-19-11-13-20(14-12-19)21-8-4-5-9-22(21)25(30)31/h4-5,8-9,11-14H,2-3,6-7,10,15-18H2,1H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50042240
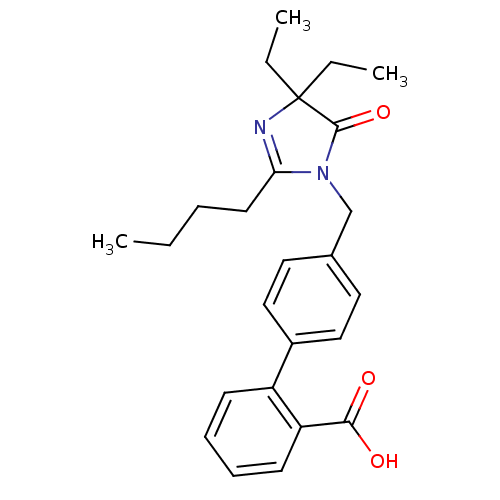
(4'-(2-Butyl-4,4-diethyl-5-oxo-4,5-dihydro-imidazol...)Show SMILES CCCCC1=NC(CC)(CC)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C25H30N2O3/c1-4-7-12-22-26-25(5-2,6-3)24(30)27(22)17-18-13-15-19(16-14-18)20-10-8-9-11-21(20)23(28)29/h8-11,13-16H,4-7,12,17H2,1-3H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421770

(CHEMBL2311099)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C Show InChI InChI=1S/C39H65N5O10/c1-22(2)17-27(30(45)20-32(47)40-25(7)35(49)41-28(18-23(3)4)31(46)21-33(48)53-11)42-37(51)34(24(5)6)44-36(50)29(19-26-15-13-12-14-16-26)43-38(52)54-39(8,9)10/h12-16,22-25,27-31,34,45-46H,17-21H2,1-11H3,(H,40,47)(H,41,49)(H,42,51)(H,43,52)(H,44,50)/t25-,27-,28-,29-,30?,31?,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282258
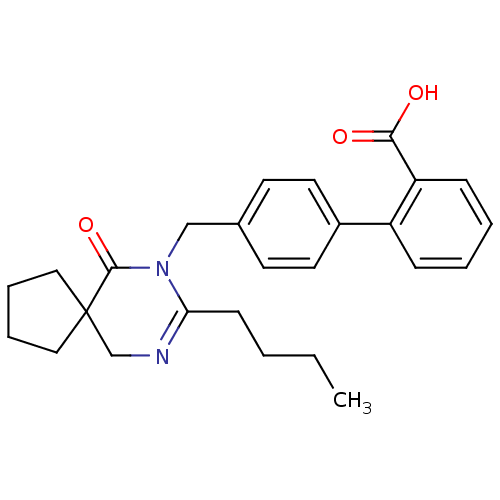
(4'-(8-Butyl-6-oxo-7,9-diaza-spiro[4.5]dec-8-en-7-y...)Show SMILES CCCCC1=NCC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C26H30N2O3/c1-2-3-10-23-27-18-26(15-6-7-16-26)25(31)28(23)17-19-11-13-20(14-12-19)21-8-4-5-9-22(21)24(29)30/h4-5,8-9,11-14H,2-3,6-7,10,15-18H2,1H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50042239
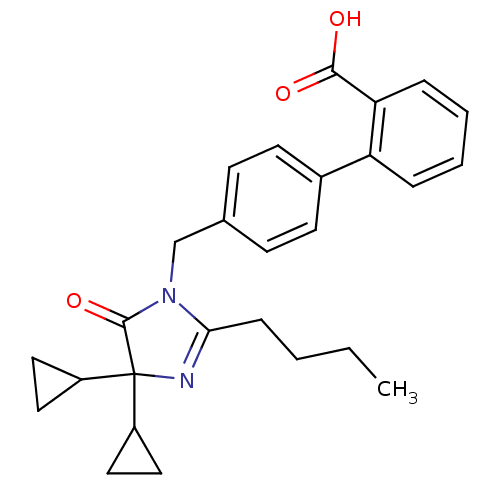
(4'-(2-Butyl-4,4-dicyclopropyl-5-oxo-4,5-dihydro-im...)Show SMILES CCCCC1=NC(C2CC2)(C2CC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C27H30N2O3/c1-2-3-8-24-28-27(20-13-14-20,21-15-16-21)26(32)29(24)17-18-9-11-19(12-10-18)22-6-4-5-7-23(22)25(30)31/h4-7,9-12,20-21H,2-3,8,13-17H2,1H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421783
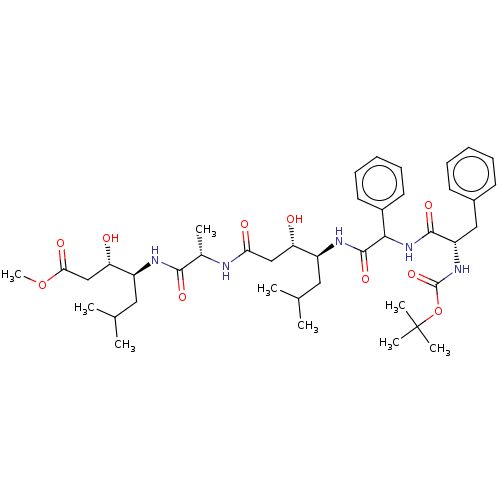
(CHEMBL3142339 | CHEMBL74979)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C42H63N5O10/c1-25(2)20-30(33(48)23-35(50)43-27(5)38(52)44-31(21-26(3)4)34(49)24-36(51)56-9)45-40(54)37(29-18-14-11-15-19-29)47-39(53)32(22-28-16-12-10-13-17-28)46-41(55)57-42(6,7)8/h10-19,25-27,30-34,37,48-49H,20-24H2,1-9H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421778
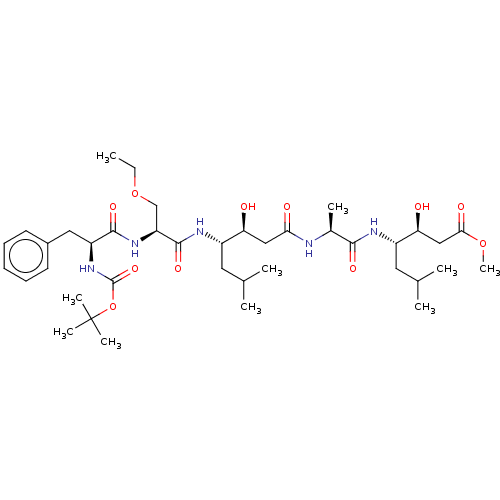
(CHEMBL3142325 | CHEMBL75458)Show SMILES CCOC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C39H65N5O11/c1-11-54-22-30(43-36(50)29(19-26-15-13-12-14-16-26)44-38(52)55-39(7,8)9)37(51)42-27(17-23(2)3)31(45)20-33(47)40-25(6)35(49)41-28(18-24(4)5)32(46)21-34(48)53-10/h12-16,23-25,27-32,45-46H,11,17-22H2,1-10H3,(H,40,47)(H,41,49)(H,42,51)(H,43,50)(H,44,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421769
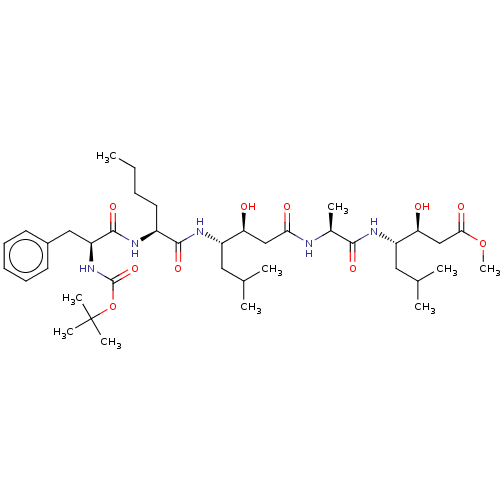
(CHEMBL3142320 | CHEMBL75210)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C40H67N5O10/c1-11-12-18-28(42-38(52)31(21-27-16-14-13-15-17-27)45-39(53)55-40(7,8)9)37(51)44-29(19-24(2)3)32(46)22-34(48)41-26(6)36(50)43-30(20-25(4)5)33(47)23-35(49)54-10/h13-17,24-26,28-33,46-47H,11-12,18-23H2,1-10H3,(H,41,48)(H,42,52)(H,43,50)(H,44,51)(H,45,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421776

(CHEMBL310597 | CHEMBL3142315)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C45H66N6O10/c1-26(2)19-33(37(52)23-39(54)47-28(5)41(56)48-34(20-27(3)4)38(53)24-40(55)60-9)49-43(58)36(22-30-25-46-32-18-14-13-17-31(30)32)50-42(57)35(21-29-15-11-10-12-16-29)51-44(59)61-45(6,7)8/h10-18,25-28,33-38,46,52-53H,19-24H2,1-9H3,(H,47,54)(H,48,56)(H,49,58)(H,50,57)(H,51,59) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024181
(4-(2-{4-[2-[2-tert-Butoxycarbonylamino-3-(1H-indol...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C42H64N8O10/c1-23(2)14-30(34(51)18-36(53)46-25(5)38(55)47-31(15-24(3)4)35(52)19-37(54)59-9)48-40(57)33(17-27-21-43-22-45-27)49-39(56)32(50-41(58)60-42(6,7)8)16-26-20-44-29-13-11-10-12-28(26)29/h10-13,20-25,30-35,44,51-52H,14-19H2,1-9H3,(H,43,45)(H,46,53)(H,47,55)(H,48,57)(H,49,56)(H,50,58) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282266
(4'-(2-Butyl-4-cyclohexylmethyl-4-methyl-5-oxo-4,5-...)Show SMILES CCCCC1=NC(C)(CC2CCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C29H36N2O3/c1-3-4-14-26-30-29(2,19-21-10-6-5-7-11-21)28(34)31(26)20-22-15-17-23(18-16-22)24-12-8-9-13-25(24)27(32)33/h8-9,12-13,15-18,21H,3-7,10-11,14,19-20H2,1-2H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022516

(Boc-Trp-Trp-Sta-Ala-Sta-OMe | CHEMBL3142766)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C Show InChI InChI=1S/C47H67N7O10/c1-26(2)18-35(39(55)22-41(57)50-28(5)43(59)51-36(19-27(3)4)40(56)23-42(58)63-9)52-44(60)37(20-29-24-48-33-16-12-10-14-31(29)33)53-45(61)38(54-46(62)64-47(6,7)8)21-30-25-49-34-17-13-11-15-32(30)34/h10-17,24-28,35-40,48-49,55-56H,18-23H2,1-9H3,(H,50,57)(H,51,59)(H,52,60)(H,53,61)(H,54,62)/t28-,35?,36?,37-,38-,39?,40?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421767
(CHEMBL312075 | CHEMBL3142324)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C1CCCCC1 |r| Show InChI InChI=1S/C42H69N5O10/c1-25(2)20-30(33(48)23-35(50)43-27(5)38(52)44-31(21-26(3)4)34(49)24-36(51)56-9)45-40(54)37(29-18-14-11-15-19-29)47-39(53)32(22-28-16-12-10-13-17-28)46-41(55)57-42(6,7)8/h10,12-13,16-17,25-27,29-34,37,48-49H,11,14-15,18-24H2,1-9H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024163

(4-[2-(4-{2-[2-Benzyloxycarbonylamino-3-(1H-indol-3...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C44H64N6O10/c1-25(2)18-33(36(51)21-38(53)46-28(7)41(55)47-34(19-26(3)4)37(52)22-39(54)59-8)48-43(57)40(27(5)6)50-42(56)35(20-30-23-45-32-17-13-12-16-31(30)32)49-44(58)60-24-29-14-10-9-11-15-29/h9-17,23,25-28,33-37,40,45,51-52H,18-22,24H2,1-8H3,(H,46,53)(H,47,55)(H,48,57)(H,49,58)(H,50,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421771

(CHEMBL307703 | CHEMBL3142321)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](COCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C44H67N5O11/c1-27(2)20-32(36(50)23-38(52)45-29(5)40(54)46-33(21-28(3)4)37(51)24-39(53)58-9)47-42(56)35(26-59-25-31-18-14-11-15-19-31)48-41(55)34(22-30-16-12-10-13-17-30)49-43(57)60-44(6,7)8/h10-19,27-29,32-37,50-51H,20-26H2,1-9H3,(H,45,52)(H,46,54)(H,47,56)(H,48,55)(H,49,57) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421761
(CHEMBL2311113)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C42H63N5O10/c1-25(2)19-31(34(48)22-36(50)43-28(7)39(52)44-32(20-26(3)4)35(49)23-37(51)56-8)45-41(54)38(27(5)6)47-40(53)33(21-29-15-11-9-12-16-29)46-42(55)57-24-30-17-13-10-14-18-30/h9-18,25-28,31-35,38,48-49H,19-24H2,1-8H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53)/t28-,31-,32-,33-,34?,35?,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024174
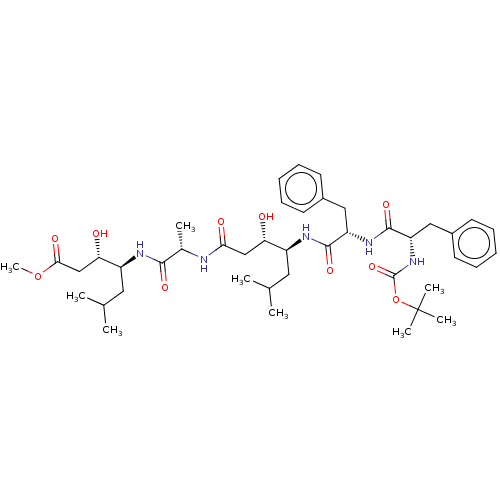
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C43H65N5O10/c1-26(2)20-31(35(49)24-37(51)44-28(5)39(53)45-32(21-27(3)4)36(50)25-38(52)57-9)46-40(54)33(22-29-16-12-10-13-17-29)47-41(55)34(23-30-18-14-11-15-19-30)48-42(56)58-43(6,7)8/h10-19,26-28,31-36,49-50H,20-25H2,1-9H3,(H,44,51)(H,45,53)(H,46,54)(H,47,55)(H,48,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282271
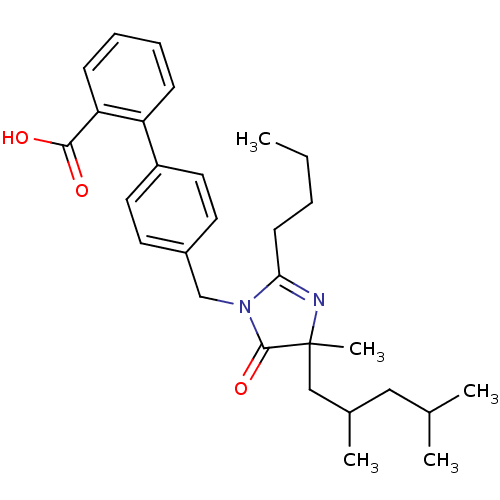
(4'-[2-Butyl-4-(2,4-dimethyl-pentyl)-4-methyl-5-oxo...)Show SMILES CCCCC1=NC(C)(CC(C)CC(C)C)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C29H38N2O3/c1-6-7-12-26-30-29(5,18-21(4)17-20(2)3)28(34)31(26)19-22-13-15-23(16-14-22)24-10-8-9-11-25(24)27(32)33/h8-11,13-16,20-21H,6-7,12,17-19H2,1-5H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421772
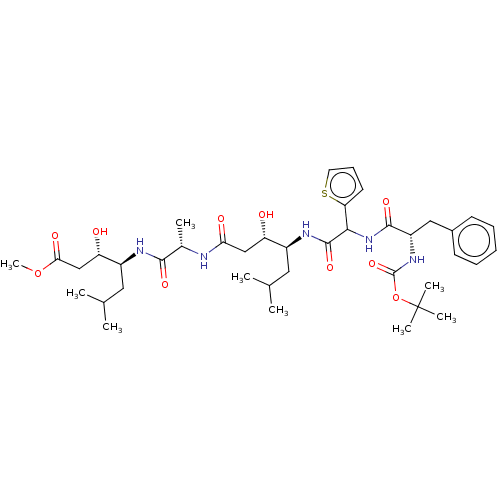
(CHEMBL3142323 | CHEMBL75470)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)c1cccs1 |r| Show InChI InChI=1S/C40H61N5O10S/c1-23(2)18-27(30(46)21-33(48)41-25(5)36(50)42-28(19-24(3)4)31(47)22-34(49)54-9)43-38(52)35(32-16-13-17-56-32)45-37(51)29(20-26-14-11-10-12-15-26)44-39(53)55-40(6,7)8/h10-17,23-25,27-31,35,46-47H,18-22H2,1-9H3,(H,41,48)(H,42,50)(H,43,52)(H,44,53)(H,45,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421768
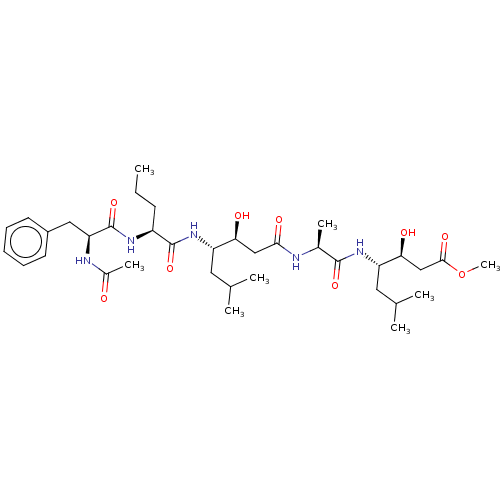
(CHEMBL3142319 | CHEMBL75987)Show SMILES CCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC |r| Show InChI InChI=1S/C36H59N5O9/c1-9-13-26(39-36(49)29(38-24(7)42)18-25-14-11-10-12-15-25)35(48)41-27(16-21(2)3)30(43)19-32(45)37-23(6)34(47)40-28(17-22(4)5)31(44)20-33(46)50-8/h10-12,14-15,21-23,26-31,43-44H,9,13,16-20H2,1-8H3,(H,37,45)(H,38,42)(H,39,49)(H,40,47)(H,41,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50421766
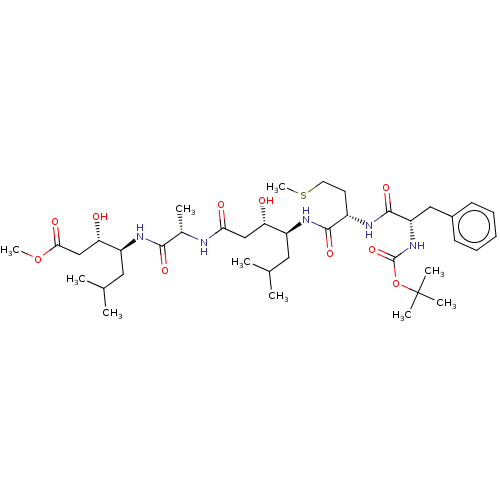
(CHEMBL3142335 | CHEMBL74682)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C39H65N5O10S/c1-23(2)18-28(31(45)21-33(47)40-25(5)35(49)42-29(19-24(3)4)32(46)22-34(48)53-9)43-36(50)27(16-17-55-10)41-37(51)30(20-26-14-12-11-13-15-26)44-38(52)54-39(6,7)8/h11-15,23-25,27-32,45-46H,16-22H2,1-10H3,(H,40,47)(H,41,51)(H,42,49)(H,43,50)(H,44,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI Recherche
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 30: 2287-91 (1988)
BindingDB Entry DOI: 10.7270/Q2D50P7G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data