

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583640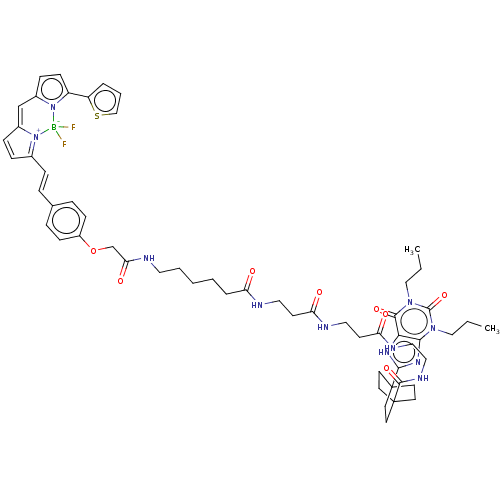 (CHEMBL5075285) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583641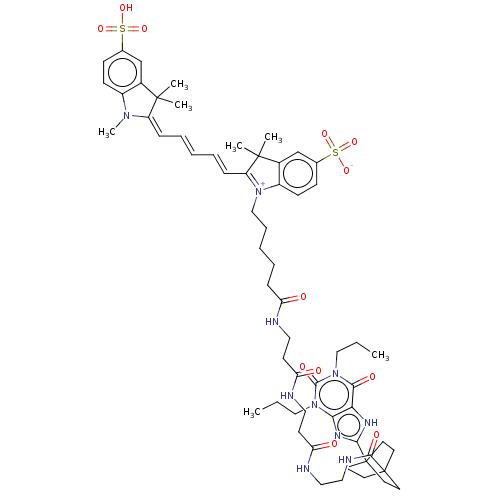 (CHEMBL5086197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of SLV320 by NanoBRET competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581950 (CHEMBL4204703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in CHO-K1 cells by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583644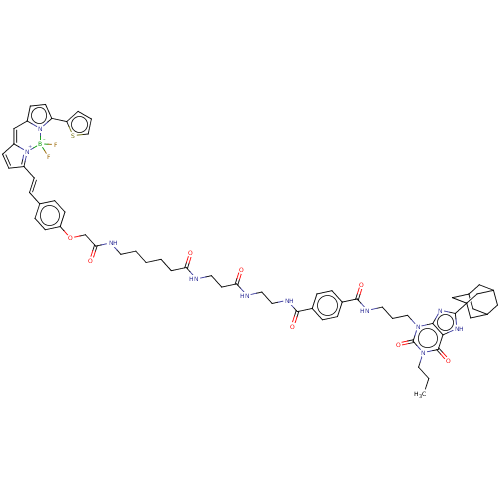 (CHEMBL5080679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of SLV320 by NanoBRET competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581950 (CHEMBL4204703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583628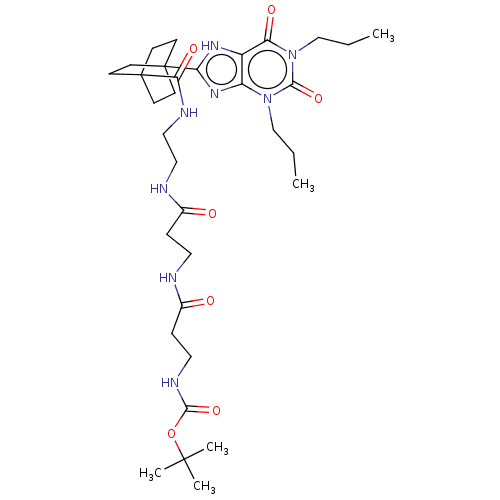 (CHEMBL5086635) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583642 (CHEMBL5080416) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583641 (CHEMBL5086197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of DPCPX by NanoBRET competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583633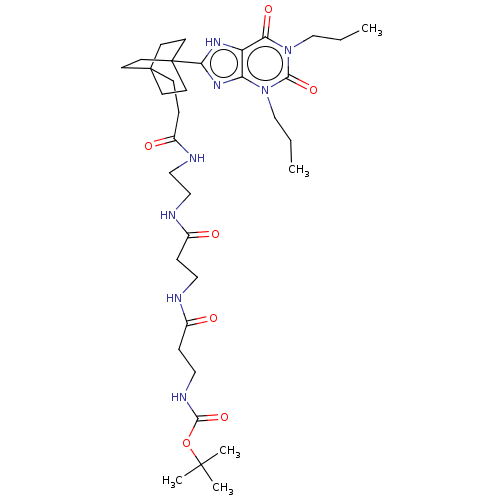 (CHEMBL5074153) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583647 (CHEMBL5081913) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to N-terminal NLuc tagged human A1 adenosine receptor expressed in Flp-In-CHO cells by competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583644 (CHEMBL5080679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583630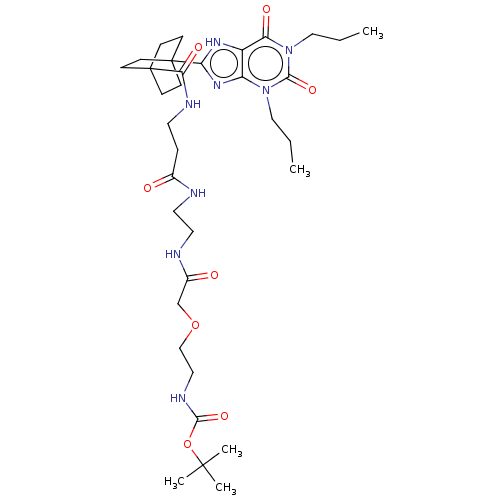 (CHEMBL5077560) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583634 (CHEMBL5090230) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583631 (CHEMBL5094274) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583629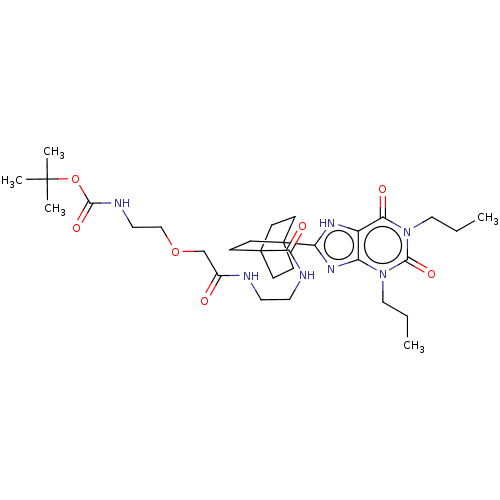 (CHEMBL5079788) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583637 (CHEMBL5081778) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583635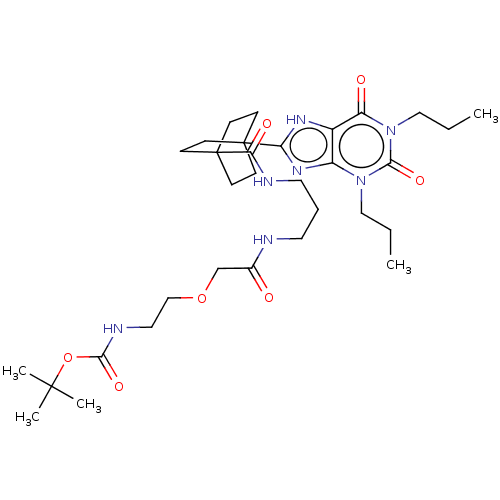 (CHEMBL5090546) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583636 (CHEMBL5078265) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583638 (CHEMBL5083466) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583644 (CHEMBL5080679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of DPCPX by NanoBRET competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583632 (CHEMBL5083938) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in CHO-K1 cells by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583641 (CHEMBL5086197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583641 (CHEMBL5086197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of NECA by NanoBRET competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583639 (CHEMBL5078024) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583644 (CHEMBL5080679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of NECA by NanoBRET competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in CHO-K1 cells by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583643 (CHEMBL5075098) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583645 (CHEMBL5074915) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50583647 (CHEMBL5081913) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at N-terminal NLuc tagged human A3 adenosine receptor | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583646 (CHEMBL5078671) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human A1 adenosine receptor | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50422942 (CHEMBL414055) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human A3 adenosine receptor expressed in Flp-In-CHO cells assessed as stimulation of cAMP accumulation by competitive binding ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50583646 (CHEMBL5078671) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at A3 adenosine receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50422943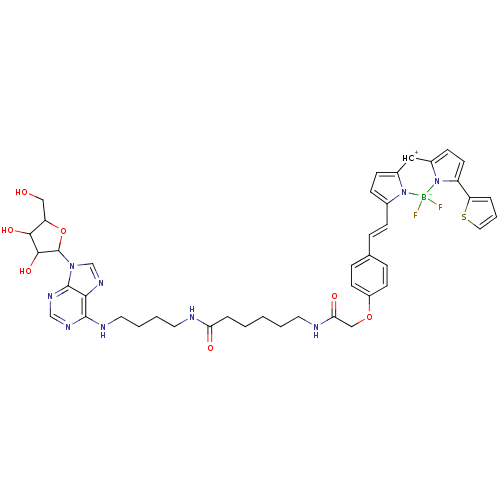 (CHEMBL375965) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at A3 adenosine receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50422943 (CHEMBL375965) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human A3 adenosine receptor expressed in Flp-In-CHO cells assessed as calcium influx by competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50583648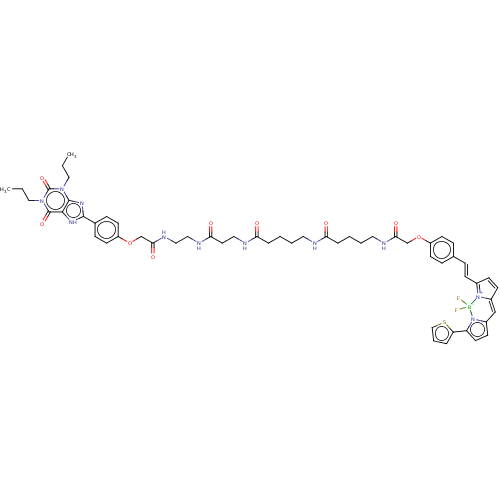 (CHEMBL5092175) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to N-terminal NLuc tagged rat A1 adenosine receptor expressed in Flp-In-CHO cells by competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50583646 (CHEMBL5078671) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to N-terminal NLuc tagged rat A1 adenosine receptor expressed in Flp-In-CHO cells by competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50422942 (CHEMBL414055) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 891 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to N-terminal NLuc tagged rat A1 adenosine receptor expressed in Flp-In-CHO cells by competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50422943 (CHEMBL375965) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 891 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to N-terminal NLuc tagged rat A1 adenosine receptor expressed in Flp-In-CHO cells by competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583648 (CHEMBL5092175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to N-terminal NLuc tagged human A1 adenosine receptor expressed in Flp-In-CHO cells by competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583646 (CHEMBL5078671) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to N-terminal NLuc tagged human A1 adenosine receptor expressed in Flp-In-CHO cells by competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50422942 (CHEMBL414055) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to N-terminal NLuc tagged human A1 adenosine receptor expressed in Flp-In-CHO cells by competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50422943 (CHEMBL375965) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 589 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to N-terminal NLuc tagged human A1 adenosine receptor expressed in Flp-In-CHO cells by competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583644 (CHEMBL5080679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to NanoLuc-human A1 adenosine receptor expressed in HEK293-A cells assessed as kinetic dissociation constant by NanoBRET competitive... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583641 (CHEMBL5086197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to NanoLuc-human A1 adenosine receptor expressed in HEK293-A cells assessed as kinetic dissociation constant by NanoBRET competitive... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50583640 (CHEMBL5075285) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to NanoLuc-human A1 adenosine receptor expressed in HEK293-A cells assessed as kinetic dissociation constant by NanoBRET competitive... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50583644 (CHEMBL5080679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to NLuc human A3 adenosine receptor expressed in HEK293-A cells in presence of 1 uM MRS1220 by NanoBRET binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50583648 (CHEMBL5092175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at N-terminal NLuc tagged human A3 adenosine receptor | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50583640 (CHEMBL5075285) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to NLuc human A3 adenosine receptor expressed in HEK293-A cells in presence of 1 uM MRS1220 by NanoBRET binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50583644 (CHEMBL5080679) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to NLuc human A2B adenosine receptor expressed in HEK293-A cells in presence of 1 uM PSB603 by NanoBRET binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02067 BindingDB Entry DOI: 10.7270/Q2CV4NM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59 total ) | Next | Last >> |