 Found 274 hits with Last Name = 'ohren' and Initial = 'jf'
Found 274 hits with Last Name = 'ohren' and Initial = 'jf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human Angiotensin receptor 1 |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human Angiotensin receptor 2 |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312
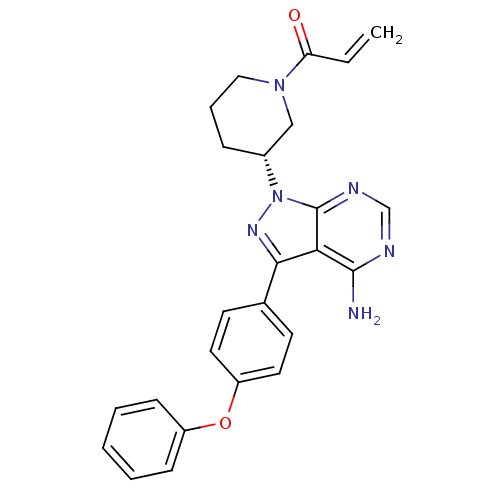
(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM377836
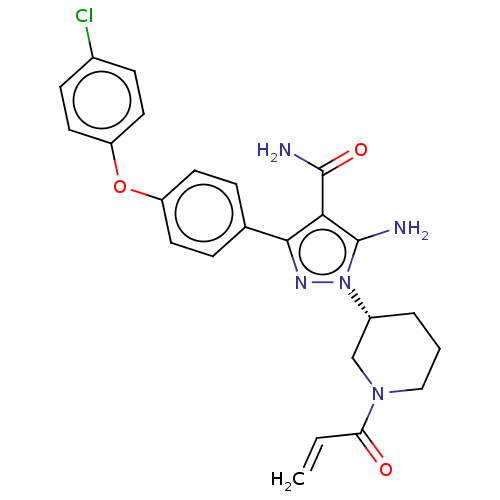
(1-[(3R)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(4...)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)[C@@H]1CCCN(C1)C(=O)C=C |r| Show InChI InChI=1S/C24H24ClN5O3/c1-2-20(31)29-13-3-4-17(14-29)30-23(26)21(24(27)32)22(28-30)15-5-9-18(10-6-15)33-19-11-7-16(25)8-12-19/h2,5-12,17H,1,3-4,13-14,26H2,(H2,27,32)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521321

(CHEMBL4442732)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(F)cc2F)cc1)[C@@H]1CCCN(C1)C(=O)C=C |r| Show InChI InChI=1S/C24H23F2N5O3/c1-2-20(32)30-11-3-4-16(13-30)31-23(27)21(24(28)33)22(29-31)14-5-8-17(9-6-14)34-19-10-7-15(25)12-18(19)26/h2,5-10,12,16H,1,3-4,11,13,27H2,(H2,28,33)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521322

(CHEMBL4436118)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccccc2)cc1)[C@@H]1CCCN(C1)C#N |r| Show InChI InChI=1S/C22H22N6O2/c23-14-27-12-4-5-16(13-27)28-21(24)19(22(25)29)20(26-28)15-8-10-18(11-9-15)30-17-6-2-1-3-7-17/h1-3,6-11,16H,4-5,12-13,24H2,(H2,25,29)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521317
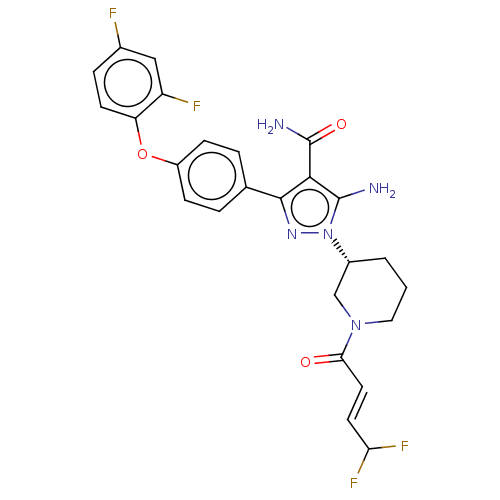
(CHEMBL4559065)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(F)cc2F)cc1)[C@@H]1CCCN(C1)C(=O)\C=C\C(F)F |r| Show InChI InChI=1S/C25H23F4N5O3/c26-15-5-8-19(18(27)12-15)37-17-6-3-14(4-7-17)23-22(25(31)36)24(30)34(32-23)16-2-1-11-33(13-16)21(35)10-9-20(28)29/h3-10,12,16,20H,1-2,11,13,30H2,(H2,31,36)/b10-9+/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50043280
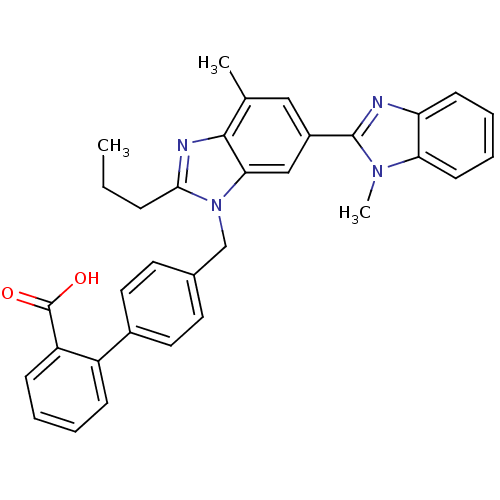
(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50512857

(CHEMBL4456283 | US10815213, Example 15)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)[C@@H]1CCCN(C1)C#N |r| Show InChI InChI=1S/C22H21ClN6O2/c23-15-5-9-18(10-6-15)31-17-7-3-14(4-8-17)20-19(22(26)30)21(25)29(27-20)16-2-1-11-28(12-16)13-24/h3-10,16H,1-2,11-12,25H2,(H2,26,30)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50222709
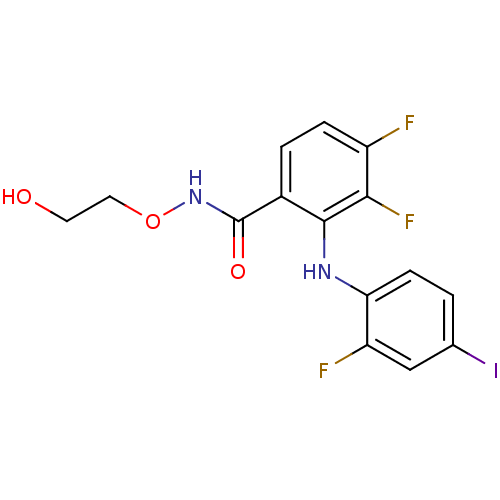
(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-N-(2-h...)Show InChI InChI=1S/C15H12F3IN2O3/c16-10-3-2-9(15(23)21-24-6-5-22)14(13(10)18)20-12-4-1-8(19)7-11(12)17/h1-4,7,20,22H,5-6H2,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of MEK assessed as inhibition of ERK phosphorylation by Raf-MEK-ERK cascade assay |
J Med Chem 50: 5090-102 (2007)
Article DOI: 10.1021/jm0704548
BindingDB Entry DOI: 10.7270/Q2474DMT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521316

(CHEMBL4560385)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(F)cc2F)cc1)[C@@H]1CCCN(C1)C#N |r| Show InChI InChI=1S/C22H20F2N6O2/c23-14-5-8-18(17(24)10-14)32-16-6-3-13(4-7-16)20-19(22(27)31)21(26)30(28-20)15-2-1-9-29(11-15)12-25/h3-8,10,15H,1-2,9,11,26H2,(H2,27,31)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521315

(CHEMBL4469663)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(Cl)cn2)cc1)[C@@H]1CCCN(C1)C#N |r| Show InChI InChI=1S/C21H20ClN7O2/c22-14-5-8-17(26-10-14)31-16-6-3-13(4-7-16)19-18(21(25)30)20(24)29(27-19)15-2-1-9-28(11-15)12-23/h3-8,10,15H,1-2,9,11,24H2,(H2,25,30)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human B cells assessed as reduction in cell proliferation pretreated for 30 mins followed... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50512857

(CHEMBL4456283 | US10815213, Example 15)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)[C@@H]1CCCN(C1)C#N |r| Show InChI InChI=1S/C22H21ClN6O2/c23-15-5-9-18(10-6-15)31-17-7-3-14(4-8-17)20-19(22(26)30)21(25)29(27-20)16-2-1-11-28(12-16)13-24/h3-10,16H,1-2,11-12,25H2,(H2,26,30)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human B cells assessed as reduction in cell proliferation pretreated for 30 mins followed... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50222709

(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-N-(2-h...)Show InChI InChI=1S/C15H12F3IN2O3/c16-10-3-2-9(15(23)21-24-6-5-22)14(13(10)18)20-12-4-1-8(19)7-11(12)17/h1-4,7,20,22H,5-6H2,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of MEK in mouse colon 26 carcinoma cells assessed as inhibition of ERK phosphorylation by ELISA |
J Med Chem 50: 5090-102 (2007)
Article DOI: 10.1021/jm0704548
BindingDB Entry DOI: 10.7270/Q2474DMT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521318

(CHEMBL4440096 | US10815213, Example 151)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(F)cc2F)cc1)[C@@H]1CCCN(C1)C(=O)\C=C\CO |r| Show InChI InChI=1S/C25H25F2N5O4/c26-16-7-10-20(19(27)13-16)36-18-8-5-15(6-9-18)23-22(25(29)35)24(28)32(30-23)17-3-1-11-31(14-17)21(34)4-2-12-33/h2,4-10,13,17,33H,1,3,11-12,14,28H2,(H2,29,35)/b4-2+/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521312
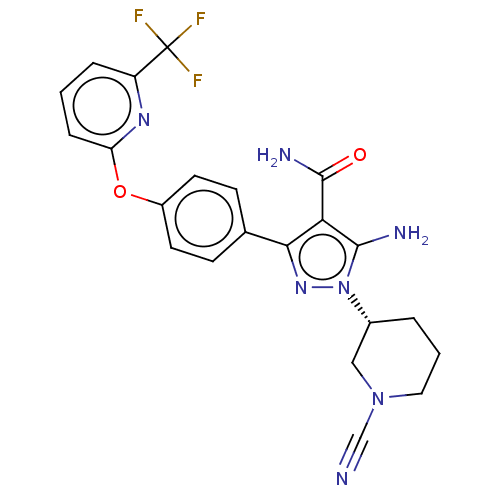
(CHEMBL4456974 | US10815213, Example 116)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2cccc(n2)C(F)(F)F)cc1)[C@@H]1CCCN(C1)C#N |r| Show InChI InChI=1S/C22H20F3N7O2/c23-22(24,25)16-4-1-5-17(29-16)34-15-8-6-13(7-9-15)19-18(21(28)33)20(27)32(30-19)14-3-2-10-31(11-14)12-26/h1,4-9,14H,2-3,10-11,27H2,(H2,28,33)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009718
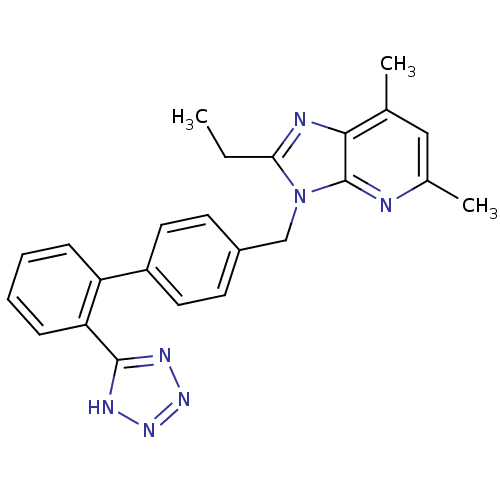
(2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7/c1-4-21-26-22-15(2)13-16(3)25-24(22)31(21)14-17-9-11-18(12-10-17)19-7-5-6-8-20(19)23-27-29-30-28-23/h5-13H,4,14H2,1-3H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521316

(CHEMBL4560385)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(F)cc2F)cc1)[C@@H]1CCCN(C1)C#N |r| Show InChI InChI=1S/C22H20F2N6O2/c23-14-5-8-18(17(24)10-14)32-16-6-3-13(4-7-16)20-19(22(27)31)21(26)30(28-20)15-2-1-9-29(11-15)12-25/h3-8,10,15H,1-2,9,11,26H2,(H2,27,31)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human B cells assessed as reduction in cell proliferation pretreated for 30 mins followed... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521314

(CHEMBL4556666 | US10815213, Example 145)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(F)cc2F)cc1)[C@@H]1CCCN(C1)C(=O)\C=C\CF |r| Show InChI InChI=1S/C25H24F3N5O3/c26-11-1-4-21(34)32-12-2-3-17(14-32)33-24(29)22(25(30)35)23(31-33)15-5-8-18(9-6-15)36-20-10-7-16(27)13-19(20)28/h1,4-10,13,17H,2-3,11-12,14,29H2,(H2,30,35)/b4-1+/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521322

(CHEMBL4436118)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccccc2)cc1)[C@@H]1CCCN(C1)C#N |r| Show InChI InChI=1S/C22H22N6O2/c23-14-27-12-4-5-16(13-27)28-21(24)19(22(25)29)20(26-28)15-8-10-18(11-9-15)30-17-6-2-1-3-7-17/h1-3,6-11,16H,4-5,12-13,24H2,(H2,25,29)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human C-terminal His6-tagged BTK C481S mutant expressed in Sf9 insect cells using FAM-Srctide peptide as substrate preincub... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM33294
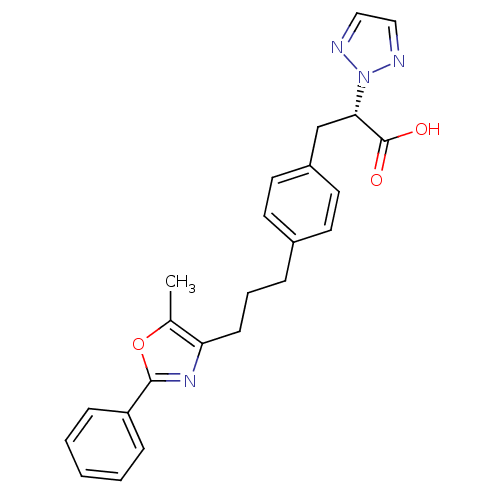
(phenylpropanoic acid derivative, 17j)Show SMILES Cc1oc(nc1CCCc1ccc(C[C@@H](C(O)=O)n2nccn2)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C24H24N4O3/c1-17-21(27-23(31-17)20-7-3-2-4-8-20)9-5-6-18-10-12-19(13-11-18)16-22(24(29)30)28-25-14-15-26-28/h2-4,7-8,10-15,22H,5-6,9,16H2,1H3,(H,29,30)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | 13 | n/a | n/a | 7.4 | 23 |
Pfizer
| Assay Description
Human 6His-PPAR ligand-binding domain was added to the mixture containing radioligand and test compound, followed by yttrium silicate polylysine SPA ... |
Bioorg Med Chem 17: 7113-25 (2009)
Article DOI: 10.1016/j.bmc.2009.09.001
BindingDB Entry DOI: 10.7270/Q2BP0158 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human C-terminal His6-tagged BTK C481S mutant expressed in Sf9 insect cells using FAM-Srctide peptide as substrate preincub... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor
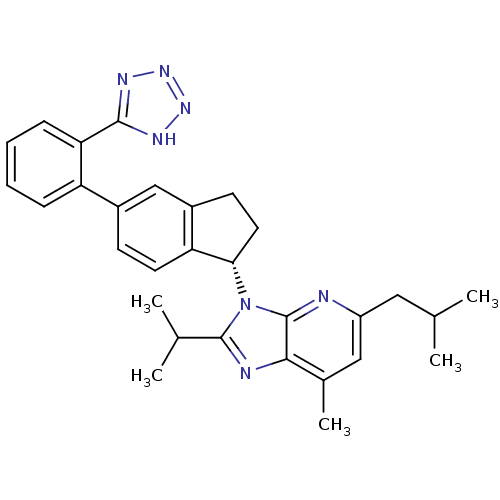
(Homo sapiens (Human)) | BDBM50347564
(CHEMBL1801741)Show SMILES CC(C)Cc1cc(C)c2nc(C(C)C)n([C@H]3CCc4cc(ccc34)-c3ccccc3-c3nnn[nH]3)c2n1 |r| Show InChI InChI=1S/C30H33N7/c1-17(2)14-22-15-19(5)27-30(31-22)37(29(32-27)18(3)4)26-13-11-21-16-20(10-12-24(21)26)23-8-6-7-9-25(23)28-33-35-36-34-28/h6-10,12,15-18,26H,11,13-14H2,1-5H3,(H,33,34,35,36)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521313

(CHEMBL4572913)Show SMILES CN(C)C\C=C\C(=O)N1CCC[C@H](C1)n1nc(c(C(N)=O)c1N)-c1ccc(Oc2ccc(F)cc2F)cc1 |r| Show InChI InChI=1S/C27H30F2N6O3/c1-33(2)13-4-6-23(36)34-14-3-5-19(16-34)35-26(30)24(27(31)37)25(32-35)17-7-10-20(11-8-17)38-22-12-9-18(28)15-21(22)29/h4,6-12,15,19H,3,5,13-14,16,30H2,1-2H3,(H2,31,37)/b6-4+/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50476830

(CHEMBL442235)Show InChI InChI=1S/C13H11FIN3O2/c1-18-6-8(13(16)20)11(5-12(18)19)17-10-3-2-7(15)4-9(10)14/h2-6,17H,1H3,(H2,16,20) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of MEK in mouse colon 26 carcinoma cells assessed as inhibition of ERK phosphorylation by ELISA |
J Med Chem 50: 5090-102 (2007)
Article DOI: 10.1021/jm0704548
BindingDB Entry DOI: 10.7270/Q2474DMT |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50521322

(CHEMBL4436118)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccccc2)cc1)[C@@H]1CCCN(C1)C#N |r| Show InChI InChI=1S/C22H22N6O2/c23-14-27-12-4-5-16(13-27)28-21(24)19(22(25)29)20(26-28)15-8-10-18(11-9-15)30-17-6-2-1-3-7-17/h1-3,6-11,16H,4-5,12-13,24H2,(H2,25,29)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length recombinant human C-terminal His-tagged SRC cytoplasmic domain expressed in baculovirus expression system using FAM-Srctide... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50476834

(CHEMBL234887)Show InChI InChI=1S/C15H15FIN3O4/c1-20-8-10(15(23)19-24-5-4-21)13(7-14(20)22)18-12-3-2-9(17)6-11(12)16/h2-3,6-8,18,21H,4-5H2,1H3,(H,19,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of MEK in mouse colon 26 carcinoma cells assessed as inhibition of ERK phosphorylation by ELISA |
J Med Chem 50: 5090-102 (2007)
Article DOI: 10.1021/jm0704548
BindingDB Entry DOI: 10.7270/Q2474DMT |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM33293

(phenylpropanoic acid derivative, 17i | racemic)Show SMILES Cc1oc(nc1CCCc1ccc(CC(C(O)=O)n2nccn2)cc1)-c1ccccc1 Show InChI InChI=1S/C24H24N4O3/c1-17-21(27-23(31-17)20-7-3-2-4-8-20)9-5-6-18-10-12-19(13-11-18)16-22(24(29)30)28-25-14-15-26-28/h2-4,7-8,10-15,22H,5-6,9,16H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | 15 | n/a | n/a | 7.4 | 23 |
Pfizer
| Assay Description
Human 6His-PPAR ligand-binding domain was added to the mixture containing radioligand and test compound, followed by yttrium silicate polylysine SPA ... |
Bioorg Med Chem 17: 7113-25 (2009)
Article DOI: 10.1016/j.bmc.2009.09.001
BindingDB Entry DOI: 10.7270/Q2BP0158 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor
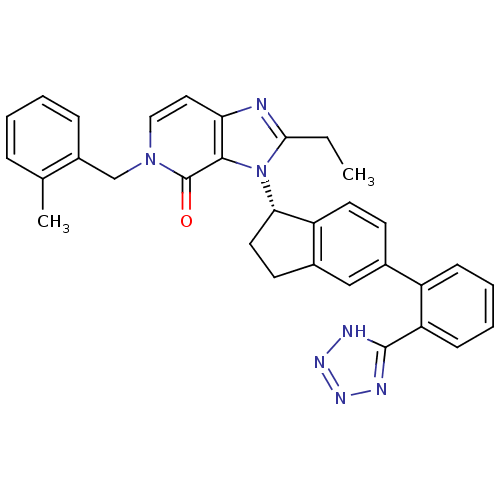
(Homo sapiens (Human)) | BDBM50426788
(CHEMBL2322444)Show SMILES CCc1nc2ccn(Cc3ccccc3C)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H29N7O/c1-3-29-33-27-16-17-38(19-23-9-5-4-8-20(23)2)32(40)30(27)39(29)28-15-13-22-18-21(12-14-25(22)28)24-10-6-7-11-26(24)31-34-36-37-35-31/h4-12,14,16-18,28H,3,13,15,19H2,1-2H3,(H,34,35,36,37)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
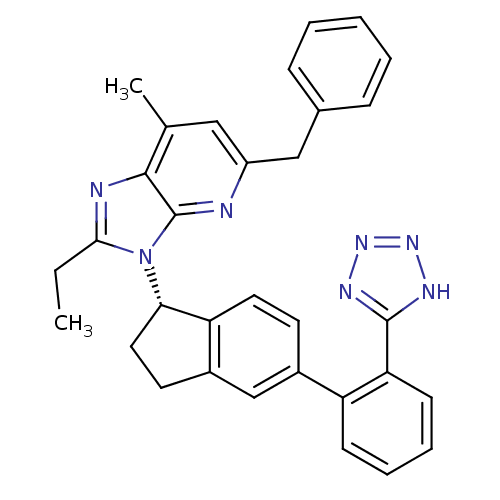
(Homo sapiens (Human)) | BDBM50347565
(CHEMBL1801743)Show SMILES CCc1nc2c(C)cc(Cc3ccccc3)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H29N7/c1-3-29-34-30-20(2)17-24(18-21-9-5-4-6-10-21)33-32(30)39(29)28-16-14-23-19-22(13-15-26(23)28)25-11-7-8-12-27(25)31-35-37-38-36-31/h4-13,15,17,19,28H,3,14,16,18H2,1-2H3,(H,35,36,37,38)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521311

(CHEMBL4591391 | US10815213, Example 150)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(F)cc2F)cc1)[C@@H]1CCCN(C1)C(=O)C(F)=C |r| Show InChI InChI=1S/C24H22F3N5O3/c1-13(25)24(34)31-10-2-3-16(12-31)32-22(28)20(23(29)33)21(30-32)14-4-7-17(8-5-14)35-19-9-6-15(26)11-18(19)27/h4-9,11,16H,1-3,10,12,28H2,(H2,29,33)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521315

(CHEMBL4469663)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(Cl)cn2)cc1)[C@@H]1CCCN(C1)C#N |r| Show InChI InChI=1S/C21H20ClN7O2/c22-14-5-8-17(26-10-14)31-16-6-3-13(4-7-16)19-18(21(25)30)20(24)29(27-19)15-2-1-9-28(11-15)12-23/h3-8,10,15H,1-2,9,11,24H2,(H2,25,30)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
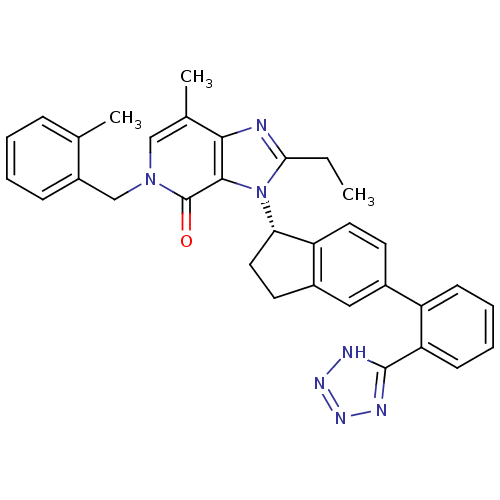
(Homo sapiens (Human)) | BDBM50426777
(CHEMBL2322175)Show SMILES CCc1nc2c(C)cn(Cc3ccccc3C)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C33H31N7O/c1-4-29-34-30-21(3)18-39(19-24-10-6-5-9-20(24)2)33(41)31(30)40(29)28-16-14-23-17-22(13-15-26(23)28)25-11-7-8-12-27(25)32-35-37-38-36-32/h5-13,15,17-18,28H,4,14,16,19H2,1-3H3,(H,35,36,37,38)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347566
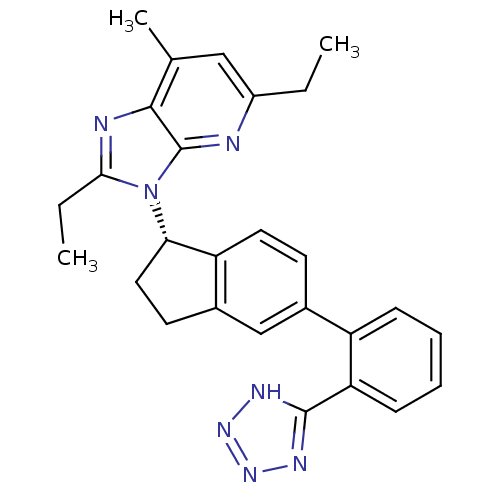
(CHEMBL1801738)Show SMILES CCc1nc2c(C)cc(CC)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C27H27N7/c1-4-19-14-16(3)25-27(28-19)34(24(5-2)29-25)23-13-11-18-15-17(10-12-21(18)23)20-8-6-7-9-22(20)26-30-32-33-31-26/h6-10,12,14-15,23H,4-5,11,13H2,1-3H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426776
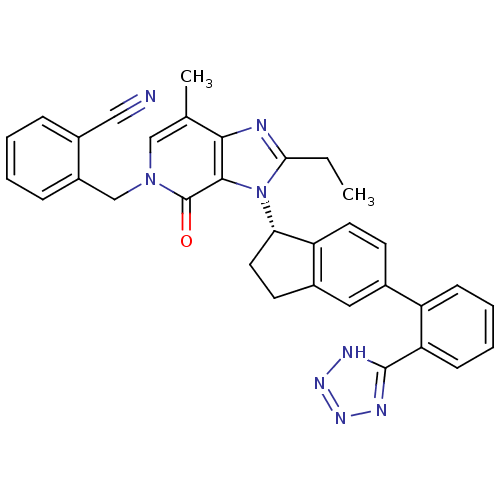
(CHEMBL2322176)Show SMILES CCc1nc2c(C)cn(Cc3ccccc3C#N)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C33H28N8O/c1-3-29-35-30-20(2)18-40(19-24-9-5-4-8-23(24)17-34)33(42)31(30)41(29)28-15-13-22-16-21(12-14-26(22)28)25-10-6-7-11-27(25)32-36-38-39-37-32/h4-12,14,16,18,28H,3,13,15,19H2,1-2H3,(H,36,37,38,39)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347567

(CHEMBL1801712)Show SMILES CCc1nc2c(C)cc(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C26H25N7/c1-4-23-28-24-15(2)13-16(3)27-26(24)33(23)22-12-10-18-14-17(9-11-20(18)22)19-7-5-6-8-21(19)25-29-31-32-30-25/h5-9,11,13-14,22H,4,10,12H2,1-3H3,(H,29,30,31,32)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM377824

((R)-5-amino-3-(4-((5-chloro-3-fluoropyridin-2-yl)o...)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ncc(Cl)cc2F)cc1)[C@@H]1CCCN(C1)C#N |r| Show InChI InChI=1S/C21H19ClFN7O2/c22-13-8-16(23)21(27-9-13)32-15-5-3-12(4-6-15)18-17(20(26)31)19(25)30(28-18)14-2-1-7-29(10-14)11-24/h3-6,8-9,14H,1-2,7,10,25H2,(H2,26,31)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His-tagged BTK expressed in baculovirus expression system using FAM-Srctide peptide as substrate preincubated for 1 h... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347568
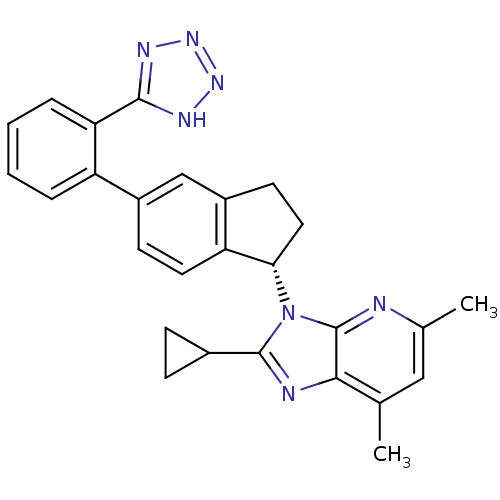
(CHEMBL1801735)Show SMILES Cc1cc(C)c2nc(C3CC3)n([C@H]3CCc4cc(ccc34)-c3ccccc3-c3nnn[nH]3)c2n1 |r| Show InChI InChI=1S/C27H25N7/c1-15-13-16(2)28-27-24(15)29-26(17-7-8-17)34(27)23-12-10-19-14-18(9-11-21(19)23)20-5-3-4-6-22(20)25-30-32-33-31-25/h3-6,9,11,13-14,17,23H,7-8,10,12H2,1-2H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347569

(CHEMBL1801734)Show SMILES CC(C)c1nc2c(C)cc(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C27H27N7/c1-15(2)26-29-24-16(3)13-17(4)28-27(24)34(26)23-12-10-19-14-18(9-11-21(19)23)20-7-5-6-8-22(20)25-30-32-33-31-25/h5-9,11,13-15,23H,10,12H2,1-4H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347570
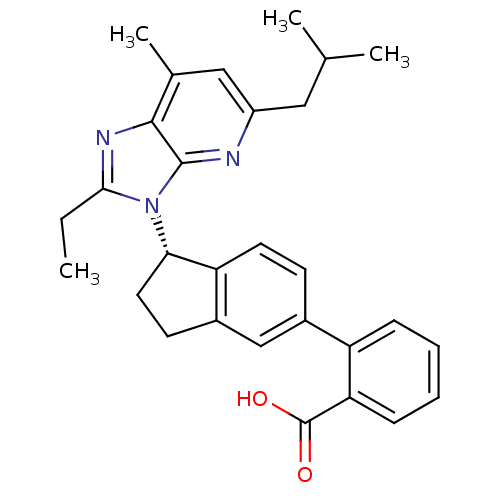
(CHEMBL1801744)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C29H31N3O2/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-28(27)32(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)29(33)34/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,33,34)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426774
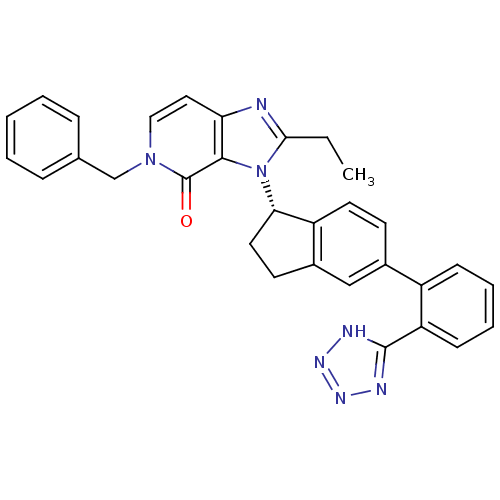
(CHEMBL2322437)Show SMILES CCc1nc2ccn(Cc3ccccc3)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C31H27N7O/c1-2-28-32-26-16-17-37(19-20-8-4-3-5-9-20)31(39)29(26)38(28)27-15-13-22-18-21(12-14-24(22)27)23-10-6-7-11-25(23)30-33-35-36-34-30/h3-12,14,16-18,27H,2,13,15,19H2,1H3,(H,33,34,35,36)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347571
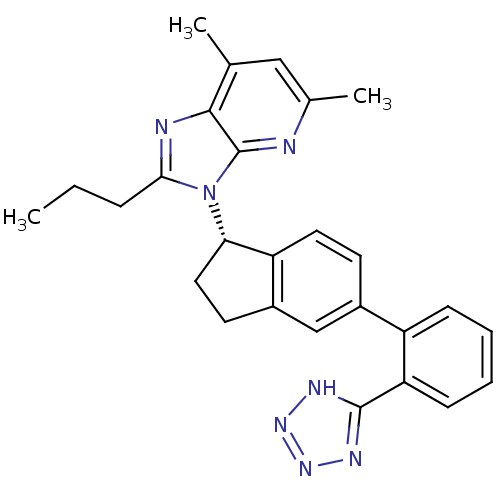
(CHEMBL1801714)Show SMILES CCCc1nc2c(C)cc(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C27H27N7/c1-4-7-24-29-25-16(2)14-17(3)28-27(25)34(24)23-13-11-19-15-18(10-12-21(19)23)20-8-5-6-9-22(20)26-30-32-33-31-26/h5-6,8-10,12,14-15,23H,4,7,11,13H2,1-3H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50426787
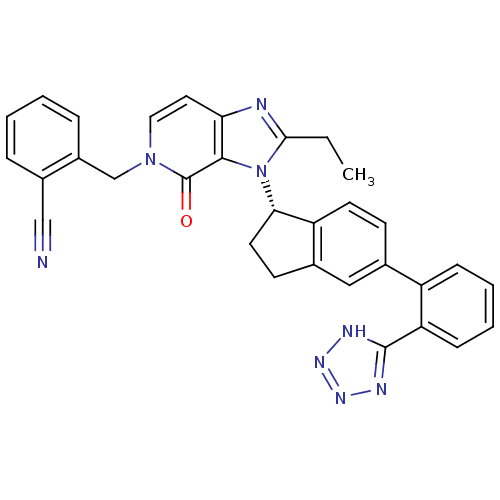
(CHEMBL2322445)Show SMILES CCc1nc2ccn(Cc3ccccc3C#N)c(=O)c2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H26N8O/c1-2-29-34-27-15-16-39(19-23-8-4-3-7-22(23)18-33)32(41)30(27)40(29)28-14-12-21-17-20(11-13-25(21)28)24-9-5-6-10-26(24)31-35-37-38-36-31/h3-11,13,15-17,28H,2,12,14,19H2,1H3,(H,35,36,37,38)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50521312

(CHEMBL4456974 | US10815213, Example 116)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2cccc(n2)C(F)(F)F)cc1)[C@@H]1CCCN(C1)C#N |r| Show InChI InChI=1S/C22H20F3N7O2/c23-22(24,25)16-4-1-5-17(29-16)34-15-8-6-13(7-9-15)19-18(21(28)33)20(27)32(30-19)14-3-2-10-31(11-14)12-26/h1,4-9,14H,2-3,10-11,27H2,(H2,28,33)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human B cells assessed as reduction in cell proliferation pretreated for 30 mins followed... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM377836

(1-[(3R)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(4...)Show SMILES NC(=O)c1c(N)n(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)[C@@H]1CCCN(C1)C(=O)C=C |r| Show InChI InChI=1S/C24H24ClN5O3/c1-2-20(31)29-13-3-4-17(14-29)30-23(26)21(24(27)32)22(28-30)15-5-9-18(10-6-15)33-19-11-7-16(25)8-12-19/h2,5-12,17H,1,3-4,13-14,26H2,(H2,27,32)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR cytoplasmic domain expressed in baculovirus expression system using FITC-C6-KKAEEEEYFELVAKK-NH2 as substrate preincubated fo... |
ACS Med Chem Lett 10: 80-85 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00461
BindingDB Entry DOI: 10.7270/Q28G8Q30 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347572

(CHEMBL1801737)Show SMILES CCc1nc2c(C)c(CC)c(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C28H29N7/c1-5-20-16(3)26-28(29-17(20)4)35(25(6-2)30-26)24-14-12-19-15-18(11-13-22(19)24)21-9-7-8-10-23(21)27-31-33-34-32-27/h7-11,13,15,24H,5-6,12,14H2,1-4H3,(H,31,32,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50132260
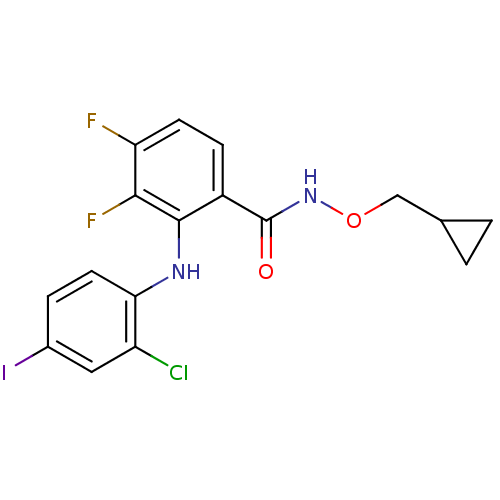
(2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmetho...)Show InChI InChI=1S/C17H14ClF2IN2O2/c18-12-7-10(21)3-6-14(12)22-16-11(4-5-13(19)15(16)20)17(24)23-25-8-9-1-2-9/h3-7,9,22H,1-2,8H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of MEK assessed as inhibition of ERK phosphorylation by Raf-MEK-ERK cascade assay |
J Med Chem 50: 5090-102 (2007)
Article DOI: 10.1021/jm0704548
BindingDB Entry DOI: 10.7270/Q2474DMT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data