

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM103511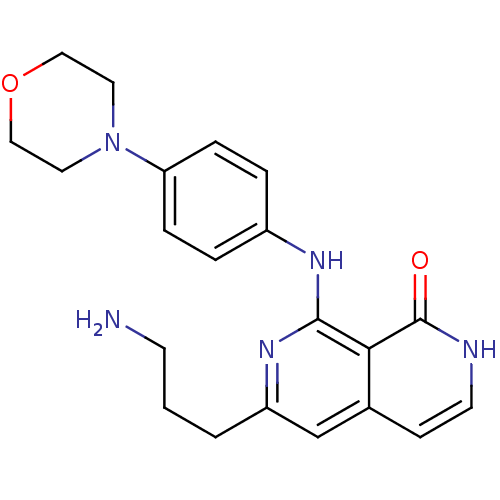 (US8546370, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRM LLC US Patent | Assay Description Homogenous time-resolved fluorescence assay using Syk enzyme. | US Patent US8546370 (2013) BindingDB Entry DOI: 10.7270/Q2GQ6WDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM103519 (US8546370, 164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
IRM LLC US Patent | Assay Description Homogenous time-resolved fluorescence assay using Syk enzyme. | US Patent US8546370 (2013) BindingDB Entry DOI: 10.7270/Q2GQ6WDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279275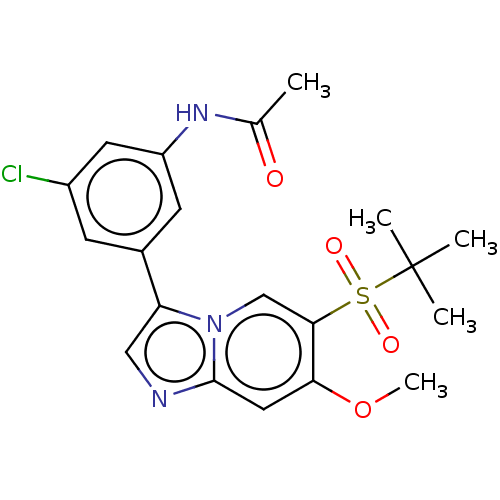 (CHEMBL4176853) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279291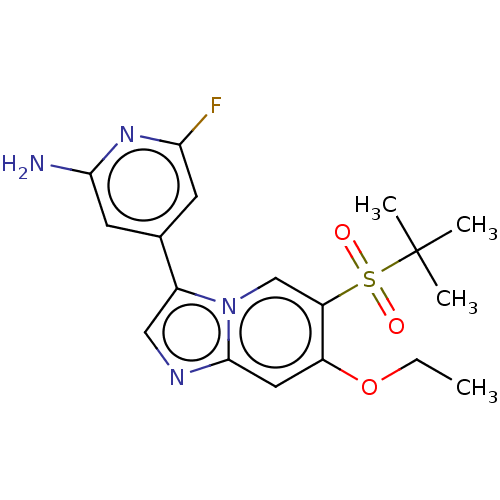 (CHEMBL4171924) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM103512 (US8546370, 83) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRM LLC US Patent | Assay Description Homogenous time-resolved fluorescence assay using Syk enzyme. | US Patent US8546370 (2013) BindingDB Entry DOI: 10.7270/Q2GQ6WDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279292 (CHEMBL4161327) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM122149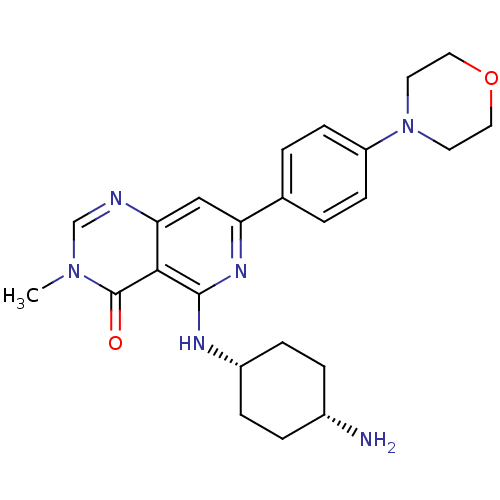 (US8722692, 589) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.89 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279311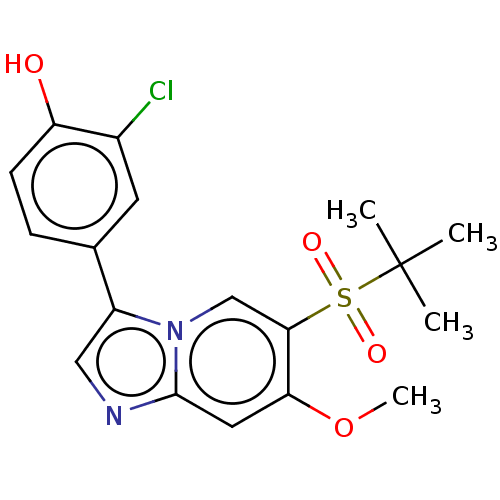 (CHEMBL4160817) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity towards nicotinic acetylcholine receptor alpha4-beta2 from rat brain homogenates | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279302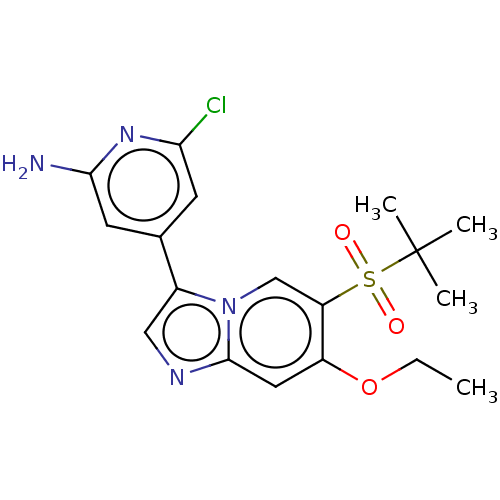 (CHEMBL4161723) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68208 (Type II inhibitor, 2) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279297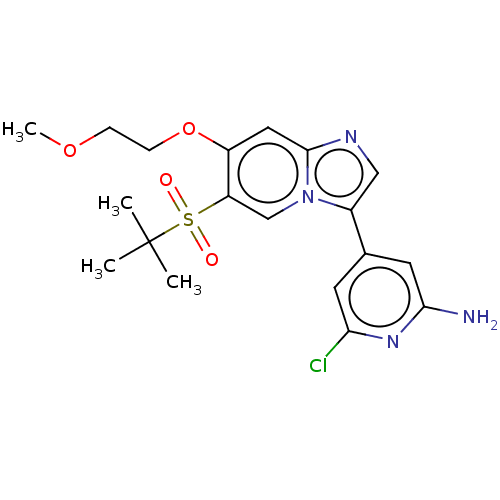 (CHEMBL4173493) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM122175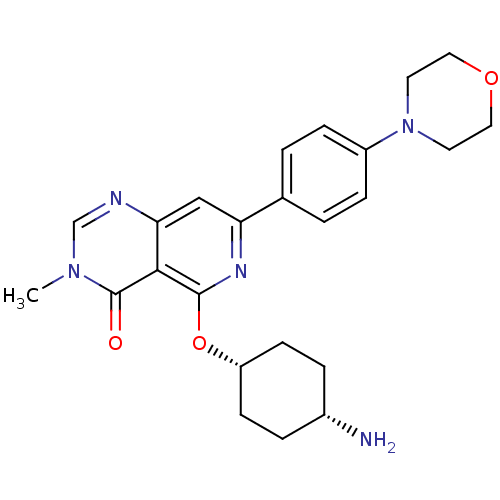 (US8722692, 615) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM122148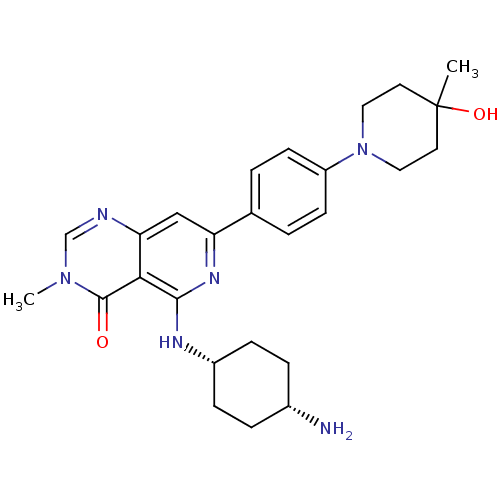 (US8722692, 588) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68217 (Type I progenitor, 11) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68217 (Type I progenitor, 11) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Mus musculus) | BDBM50279297 (CHEMBL4173493) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of RIPK2 in C57BL/6 mouse BMDM assessed as reduction in MDP/LPS-stimulated IL6 secretion pretreated for 30 mins followed by MDP/LPS stimul... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279317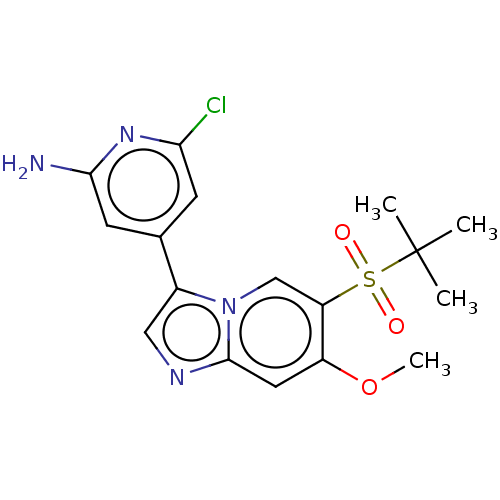 (CHEMBL4166239) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM121722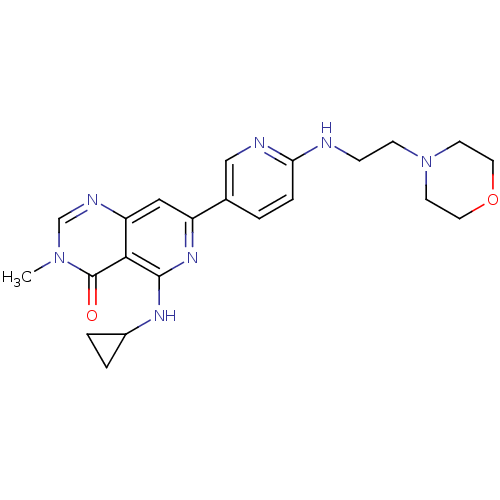 (US8722692, 161) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <6.86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279303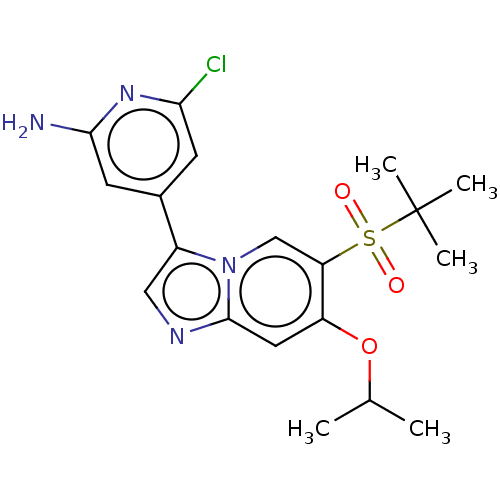 (CHEMBL4170048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279325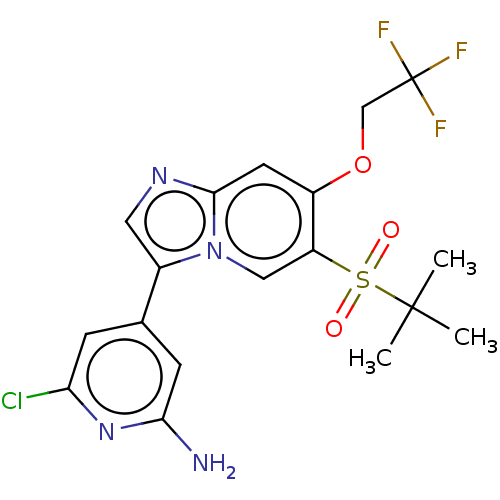 (CHEMBL4159424) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279296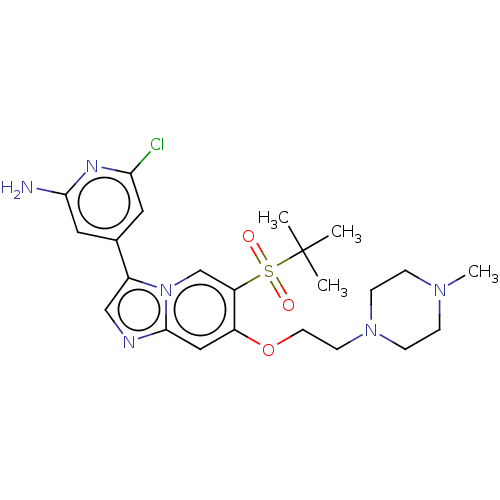 (CHEMBL4162788) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM103524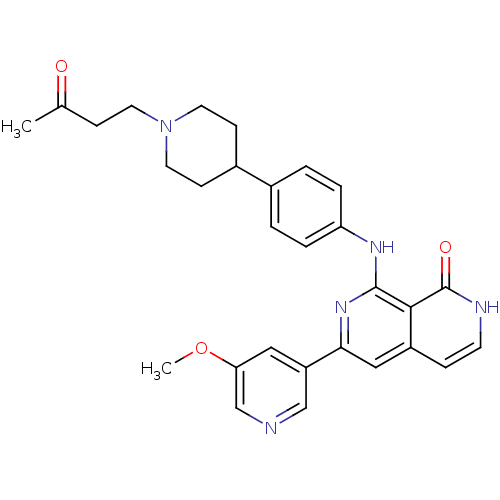 (US8546370, 304) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
IRM LLC US Patent | Assay Description Homogenous time-resolved fluorescence assay using Syk enzyme. | US Patent US8546370 (2013) BindingDB Entry DOI: 10.7270/Q2GQ6WDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM103522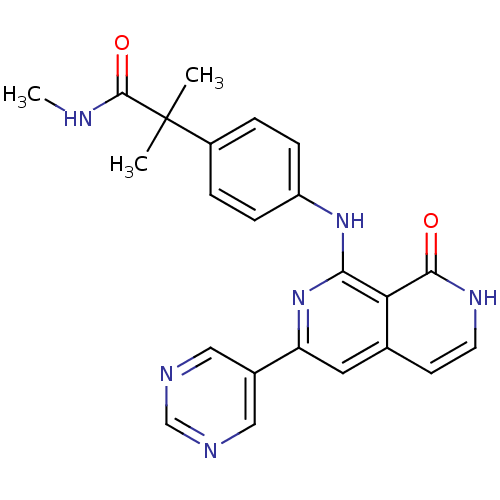 (US8546370, 190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
IRM LLC US Patent | Assay Description Homogenous time-resolved fluorescence assay using Syk enzyme. | US Patent US8546370 (2013) BindingDB Entry DOI: 10.7270/Q2GQ6WDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM50242740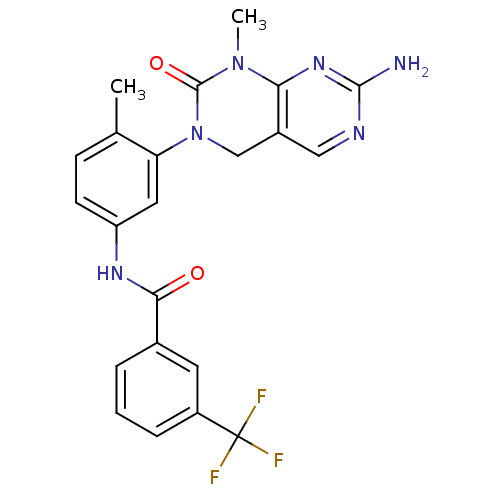 (CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279313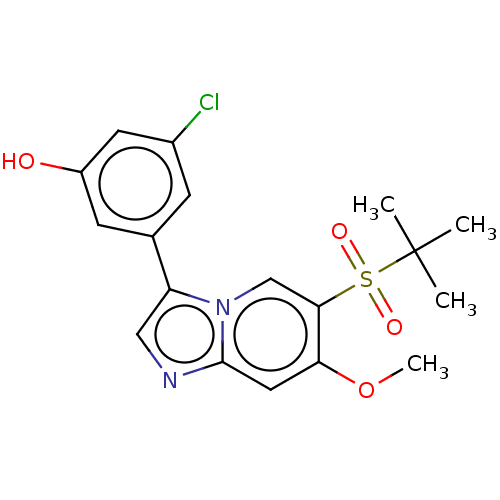 (CHEMBL4164217) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279317 (CHEMBL4166239) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of RIPK2 in human PBMCs assessed as reduction in MDP-stimulated IL8 secretion measured after 18 hrs | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM103512 (US8546370, 83) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRM LLC US Patent | Assay Description Homogenous time-resolved fluorescence assay using Syk enzyme. | US Patent US8546370 (2013) BindingDB Entry DOI: 10.7270/Q2GQ6WDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279275 (CHEMBL4176853) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of RIPK2 in human PBMCs assessed as reduction in MDP-stimulated IL8 secretion measured after 18 hrs | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68219 (Type II inhibitor, 13) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM121563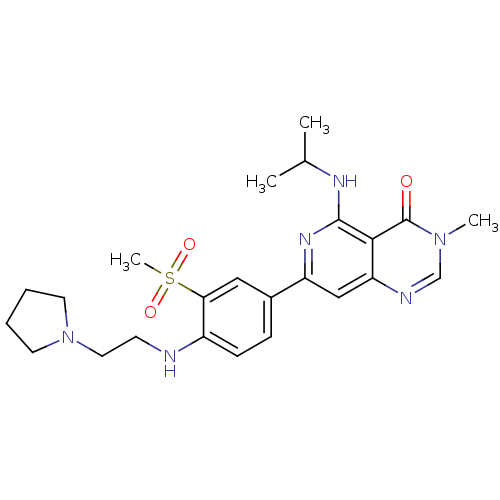 (US8722692, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279323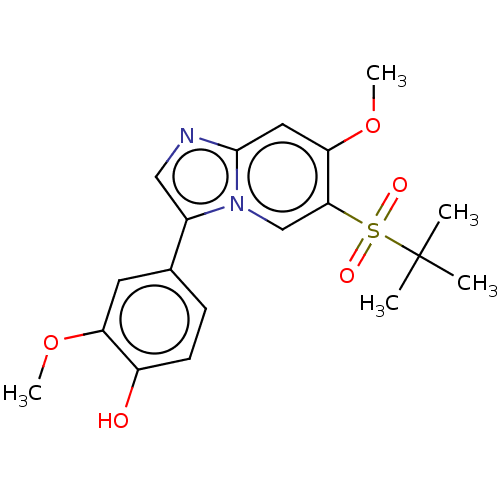 (CHEMBL4168738) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM121648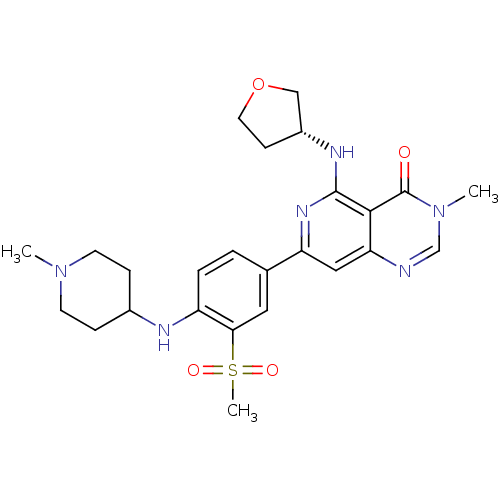 (US8722692, 87) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM50242740 (CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Mus musculus) | BDBM50279292 (CHEMBL4161327) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of RIPK2 in C57BL/6 mouse BMDM assessed as reduction in MDP/LPS-stimulated IL6 secretion pretreated for 30 mins followed by MDP/LPS stimul... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM121562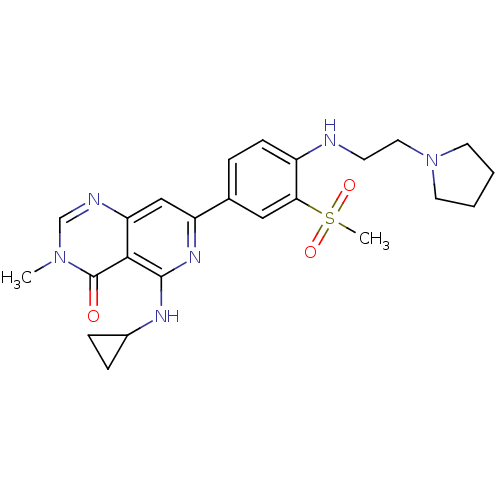 (US8722692, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279320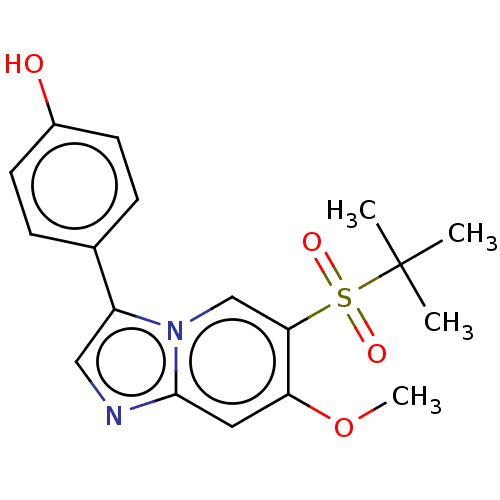 (CHEMBL4163484) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM122034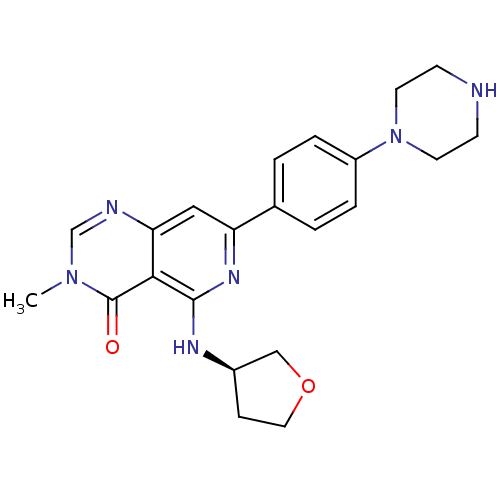 (US8722692, 473) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM122046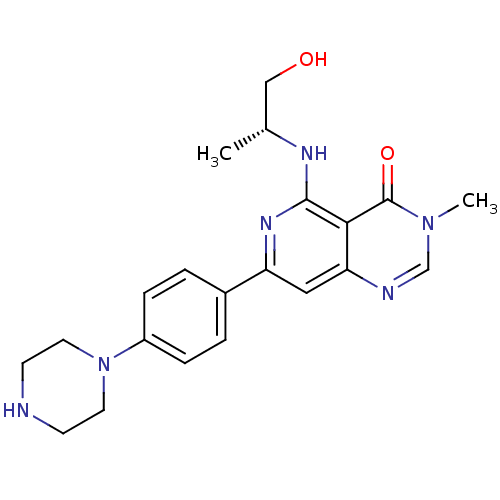 (US8722692, 485) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68218 (Type II inhibitor, 12) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19.6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279316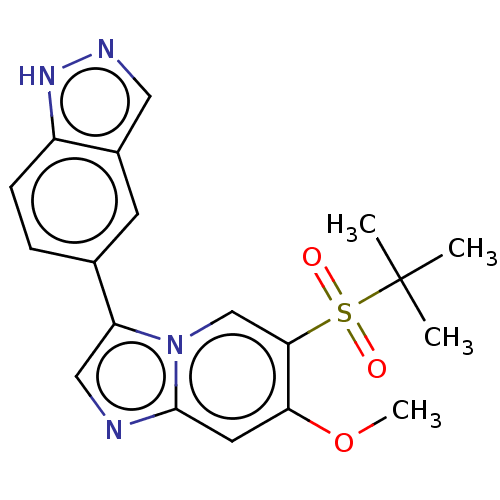 (CHEMBL4167668) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM121641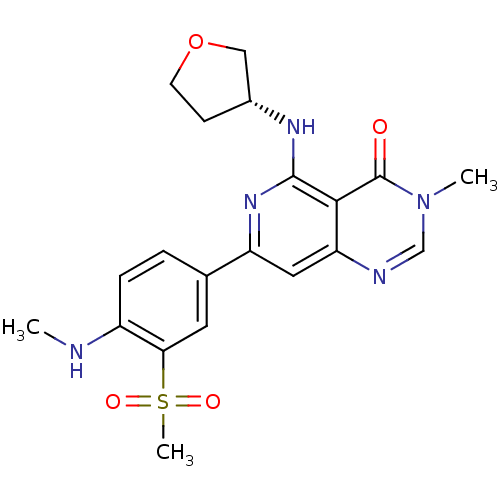 (US8722692, 80) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279290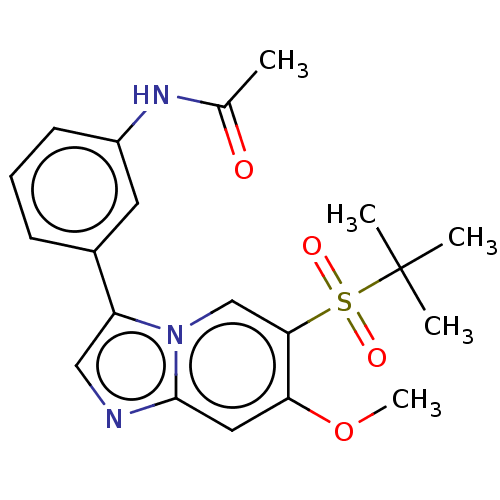 (CHEMBL4171039) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM6568 (6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM121951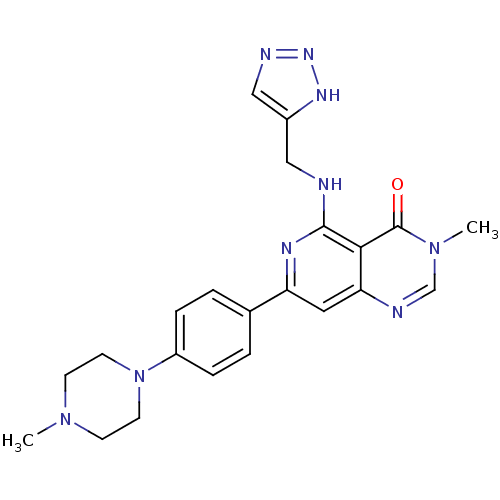 (US8722692, 390) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM6568 (6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50279293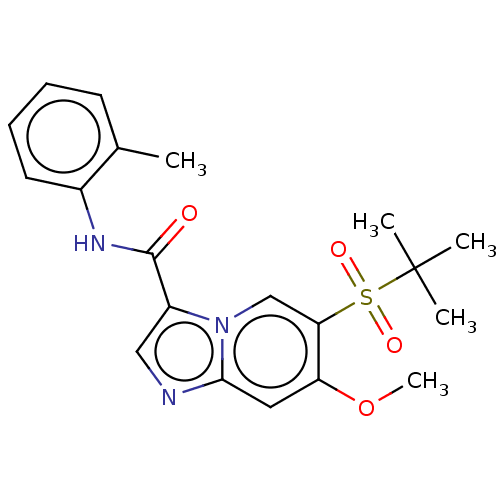 (CHEMBL4162913) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of full length RIPK2 (unknown origin) pretreated for 30 mins followed by ATP addition measured after 2 hrs by ADP-d2 tracer based fluoresc... | ACS Med Chem Lett 8: 1048-1053 (2017) Article DOI: 10.1021/acsmedchemlett.7b00258 BindingDB Entry DOI: 10.7270/Q2ST7SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM103521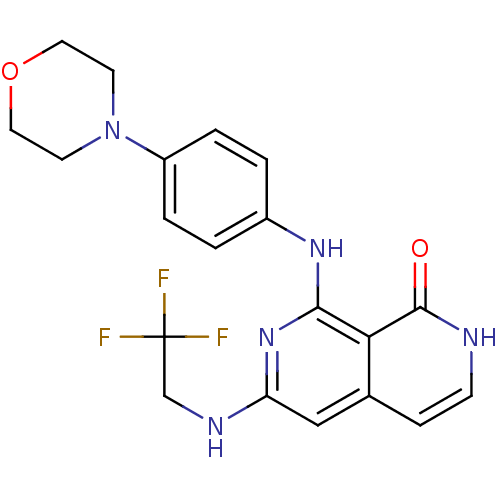 (US8546370, 183) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
IRM LLC US Patent | Assay Description Homogenous time-resolved fluorescence assay using Syk enzyme. | US Patent US8546370 (2013) BindingDB Entry DOI: 10.7270/Q2GQ6WDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM122061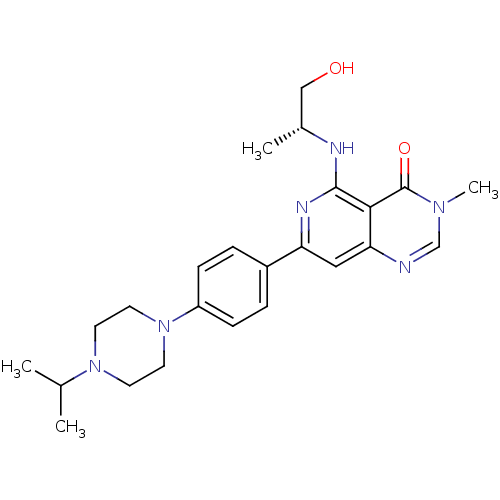 (US8722692, 500) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM122181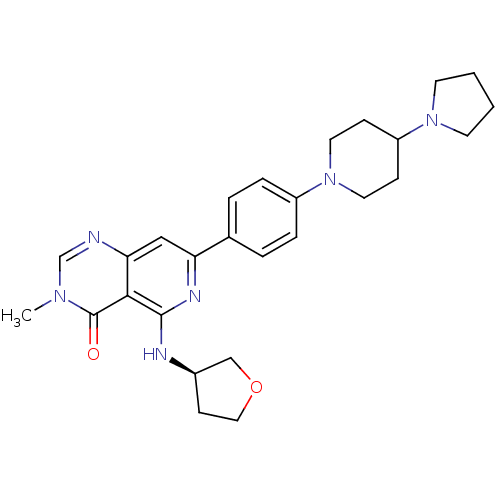 (US8722692, 621) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM122178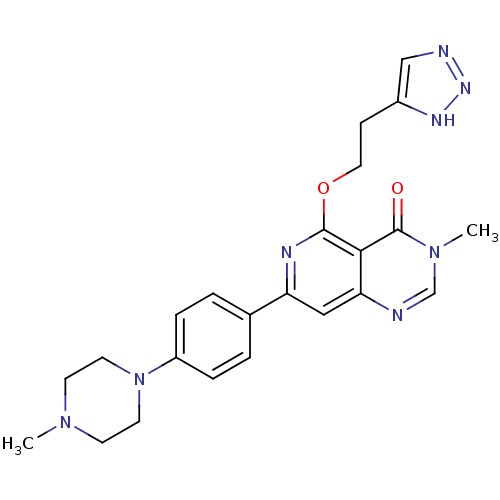 (US8722692, 618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary L... | US Patent US8722692 (2014) BindingDB Entry DOI: 10.7270/Q24Q7SNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1336 total ) | Next | Last >> |