 Found 121 hits with Last Name = 'oleksyszyn' and Initial = 'j'
Found 121 hits with Last Name = 'oleksyszyn' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50244147

((4-methoxybenzyl)carbamothioyl(1-aminopentyl)phosp...)Show InChI InChI=1S/C14H23N2O3PS/c1-3-4-5-13(15)20(17,18)14(21)16-10-11-6-8-12(19-2)9-7-11/h6-9,17-18H,3-5,10,15H2,1-2H3,(H,16,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N |
Bioorg Med Chem Lett 18: 3734-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.050
BindingDB Entry DOI: 10.7270/Q2H41R6T |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Homo sapiens (Human)) | BDBM50316047
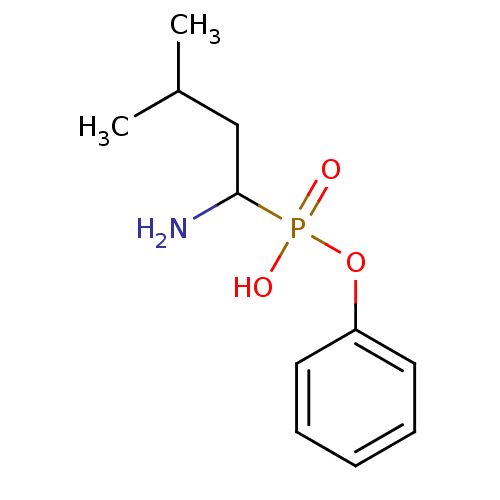
(1-amino-2-methylbutylphosphonic acid monophenylest...)Show InChI InChI=1S/C11H18NO3P/c1-9(2)8-11(12)16(13,14)15-10-6-4-3-5-7-10/h3-7,9,11H,8,12H2,1-2H3,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of leucine aminopeptidase |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Homo sapiens (Human)) | BDBM50129679
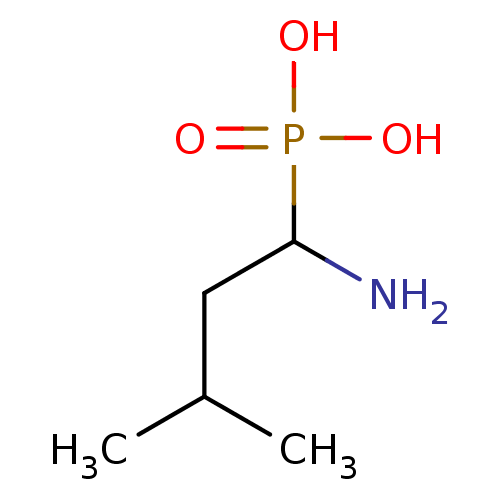
((1-Amino-3-methyl-butyl)-phosphonic acid | 1-amino...)Show InChI InChI=1S/C5H14NO3P/c1-4(2)3-5(6)10(7,8)9/h4-5H,3,6H2,1-2H3,(H2,7,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of leucine aminopeptidase |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Homo sapiens (Human)) | BDBM50316048

(1-amino-2-phenylethylphosphonic acid monophenyl es...)Show InChI InChI=1S/C14H16NO3P/c15-14(11-12-7-3-1-4-8-12)19(16,17)18-13-9-5-2-6-10-13/h1-10,14H,11,15H2,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of leucine aminopeptidase |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Homo sapiens (Human)) | BDBM50024592

((S)-1-Ammonium-2-phenyl-ethanephosphonic acid anio...)Show InChI InChI=1S/C8H12NO3P/c9-8(13(10,11)12)6-7-4-2-1-3-5-7/h1-5,8H,6,9H2,(H2,10,11,12)/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of leucine aminopeptidase |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Glutamyl endopeptidase
(Staphylococcus aureus) | BDBM50435121
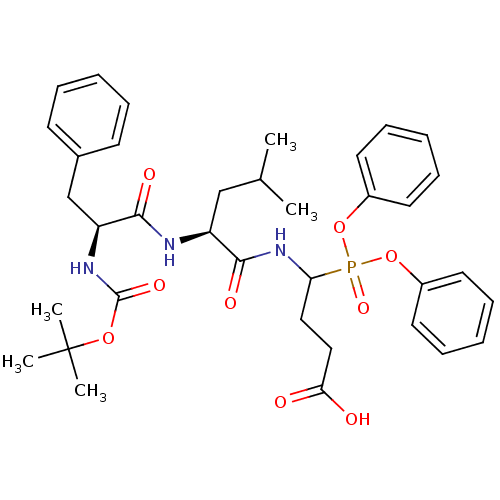
(CHEMBL2391716)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NC(CCC(O)=O)P(=O)(Oc1ccccc1)Oc1ccccc1 |r| Show InChI InChI=1S/C36H46N3O9P/c1-25(2)23-29(37-33(42)30(24-26-15-9-6-10-16-26)38-35(44)46-36(3,4)5)34(43)39-31(21-22-32(40)41)49(45,47-27-17-11-7-12-18-27)48-28-19-13-8-14-20-28/h6-20,25,29-31H,21-24H2,1-5H3,(H,37,42)(H,38,44)(H,39,43)(H,40,41)/t29-,30-,31?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus endoproteinase GluC using Ac-Phe-Leu-Glu-ACC as substrate after 30 mins |
Bioorg Med Chem Lett 23: 1412-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.074
BindingDB Entry DOI: 10.7270/Q2SX6FKB |
More data for this
Ligand-Target Pair | |
Glutamyl endopeptidase
(Staphylococcus aureus) | BDBM50435123
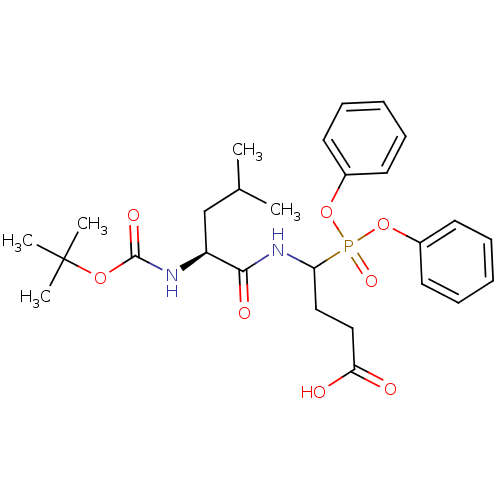
(CHEMBL2391713)Show SMILES CC(C)C[C@H](NC(=O)OC(C)(C)C)C(=O)NC(CCC(O)=O)P(=O)(Oc1ccccc1)Oc1ccccc1 |r| Show InChI InChI=1S/C27H37N2O8P/c1-19(2)18-22(28-26(33)35-27(3,4)5)25(32)29-23(16-17-24(30)31)38(34,36-20-12-8-6-9-13-20)37-21-14-10-7-11-15-21/h6-15,19,22-23H,16-18H2,1-5H3,(H,28,33)(H,29,32)(H,30,31)/t22-,23?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus endoproteinase GluC using Ac-Phe-Leu-Glu-ACC as substrate after 30 mins |
Bioorg Med Chem Lett 23: 1412-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.074
BindingDB Entry DOI: 10.7270/Q2SX6FKB |
More data for this
Ligand-Target Pair | |
Glutamyl endopeptidase
(Staphylococcus aureus) | BDBM50435124
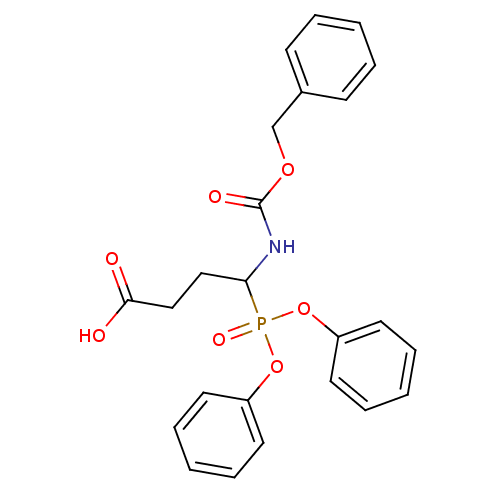
(CHEMBL2390964)Show SMILES OC(=O)CCC(NC(=O)OCc1ccccc1)P(=O)(Oc1ccccc1)Oc1ccccc1 Show InChI InChI=1S/C24H24NO7P/c26-23(27)17-16-22(25-24(28)30-18-19-10-4-1-5-11-19)33(29,31-20-12-6-2-7-13-20)32-21-14-8-3-9-15-21/h1-15,22H,16-18H2,(H,25,28)(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus endoproteinase GluC using Ac-Phe-Leu-Glu-ACC as substrate after 30 mins |
Bioorg Med Chem Lett 23: 1412-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.074
BindingDB Entry DOI: 10.7270/Q2SX6FKB |
More data for this
Ligand-Target Pair | |
Glutamyl endopeptidase
(Staphylococcus aureus) | BDBM50435117
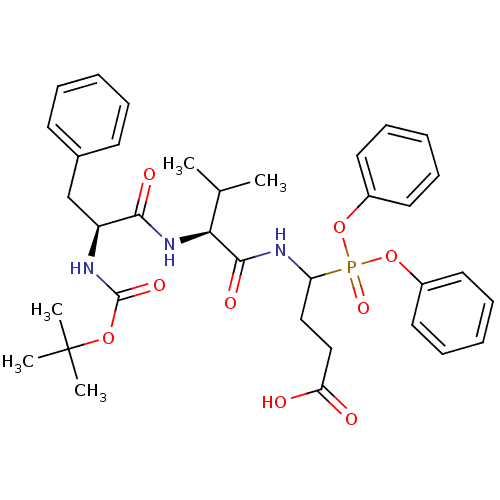
(CHEMBL2391722)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NC(CCC(O)=O)P(=O)(Oc1ccccc1)Oc1ccccc1 |r| Show InChI InChI=1S/C35H44N3O9P/c1-24(2)31(38-32(41)28(23-25-15-9-6-10-16-25)36-34(43)45-35(3,4)5)33(42)37-29(21-22-30(39)40)48(44,46-26-17-11-7-12-18-26)47-27-19-13-8-14-20-27/h6-20,24,28-29,31H,21-23H2,1-5H3,(H,36,43)(H,37,42)(H,38,41)(H,39,40)/t28-,29?,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus endoproteinase GluC using Ac-Phe-Leu-Glu-ACC as substrate after 30 mins |
Bioorg Med Chem Lett 23: 1412-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.074
BindingDB Entry DOI: 10.7270/Q2SX6FKB |
More data for this
Ligand-Target Pair | |
Glutamyl endopeptidase
(Staphylococcus aureus) | BDBM50435119
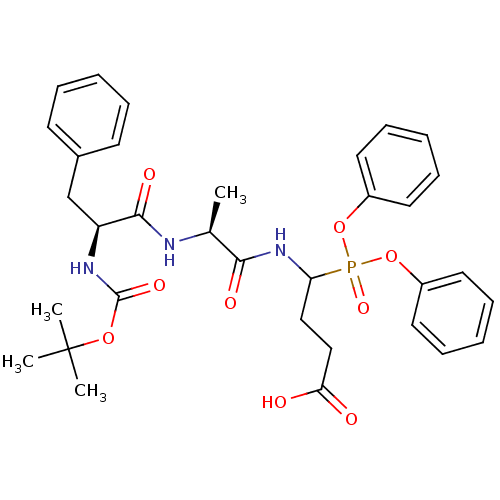
(CHEMBL2391719)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NC(CCC(O)=O)P(=O)(Oc1ccccc1)Oc1ccccc1 |r| Show InChI InChI=1S/C33H40N3O9P/c1-23(34-31(40)27(22-24-14-8-5-9-15-24)35-32(41)43-33(2,3)4)30(39)36-28(20-21-29(37)38)46(42,44-25-16-10-6-11-17-25)45-26-18-12-7-13-19-26/h5-19,23,27-28H,20-22H2,1-4H3,(H,34,40)(H,35,41)(H,36,39)(H,37,38)/t23-,27-,28?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus endoproteinase GluC using Ac-Phe-Leu-Glu-ACC as substrate after 30 mins |
Bioorg Med Chem Lett 23: 1412-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.074
BindingDB Entry DOI: 10.7270/Q2SX6FKB |
More data for this
Ligand-Target Pair | |
Glutamyl endopeptidase
(Staphylococcus aureus) | BDBM50435120
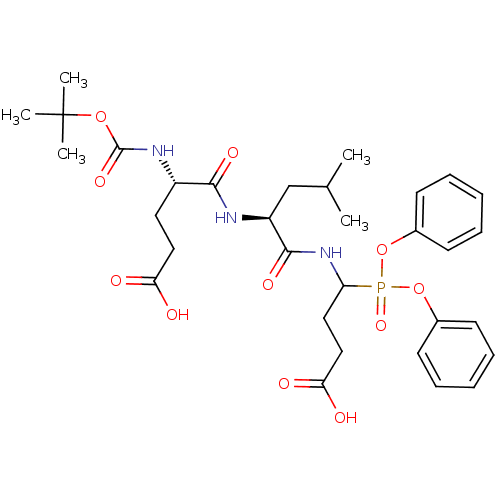
(CHEMBL2391718)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)OC(C)(C)C)C(=O)NC(CCC(O)=O)P(=O)(Oc1ccccc1)Oc1ccccc1 |r| Show InChI InChI=1S/C32H44N3O11P/c1-21(2)20-25(33-29(40)24(16-18-27(36)37)34-31(42)44-32(3,4)5)30(41)35-26(17-19-28(38)39)47(43,45-22-12-8-6-9-13-22)46-23-14-10-7-11-15-23/h6-15,21,24-26H,16-20H2,1-5H3,(H,33,40)(H,34,42)(H,35,41)(H,36,37)(H,38,39)/t24-,25-,26?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus endoproteinase GluC using Ac-Phe-Leu-Glu-ACC as substrate after 30 mins |
Bioorg Med Chem Lett 23: 1412-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.074
BindingDB Entry DOI: 10.7270/Q2SX6FKB |
More data for this
Ligand-Target Pair | |
Glutamyl endopeptidase
(Staphylococcus aureus) | BDBM50435116
(CHEMBL2391724)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)OC(C)(C)C)C(=O)NC(CCC(O)=O)P(=O)(Oc1ccccc1)Oc1ccccc1 |r| Show InChI InChI=1S/C31H42N3O11P/c1-20(2)27(34-28(39)23(16-18-25(35)36)32-30(41)43-31(3,4)5)29(40)33-24(17-19-26(37)38)46(42,44-21-12-8-6-9-13-21)45-22-14-10-7-11-15-22/h6-15,20,23-24,27H,16-19H2,1-5H3,(H,32,41)(H,33,40)(H,34,39)(H,35,36)(H,37,38)/t23-,24?,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus endoproteinase GluC using Ac-Phe-Leu-Glu-ACC as substrate after 30 mins |
Bioorg Med Chem Lett 23: 1412-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.074
BindingDB Entry DOI: 10.7270/Q2SX6FKB |
More data for this
Ligand-Target Pair | |
Glutamyl endopeptidase
(Staphylococcus aureus) | BDBM50435125

(CHEMBL2391726)Show SMILES CC(=O)NC(CCC(O)=O)P(=O)(Oc1ccccc1)Oc1ccccc1 Show InChI InChI=1S/C18H20NO6P/c1-14(20)19-17(12-13-18(21)22)26(23,24-15-8-4-2-5-9-15)25-16-10-6-3-7-11-16/h2-11,17H,12-13H2,1H3,(H,19,20)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus endoproteinase GluC using Ac-Phe-Leu-Glu-ACC as substrate after 30 mins |
Bioorg Med Chem Lett 23: 1412-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.074
BindingDB Entry DOI: 10.7270/Q2SX6FKB |
More data for this
Ligand-Target Pair | |
Glutamyl endopeptidase
(Staphylococcus aureus) | BDBM50435118

(CHEMBL2391721)Show SMILES C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)OC(C)(C)C)C(=O)NC(CCC(O)=O)P(=O)(Oc1ccccc1)Oc1ccccc1 |r| Show InChI InChI=1S/C29H38N3O11P/c1-19(30-27(38)22(15-17-24(33)34)31-28(39)41-29(2,3)4)26(37)32-23(16-18-25(35)36)44(40,42-20-11-7-5-8-12-20)43-21-13-9-6-10-14-21/h5-14,19,22-23H,15-18H2,1-4H3,(H,30,38)(H,31,39)(H,32,37)(H,33,34)(H,35,36)/t19-,22-,23?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus endoproteinase GluC using Ac-Phe-Leu-Glu-ACC as substrate after 30 mins |
Bioorg Med Chem Lett 23: 1412-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.074
BindingDB Entry DOI: 10.7270/Q2SX6FKB |
More data for this
Ligand-Target Pair | |
Glutamyl endopeptidase
(Staphylococcus aureus) | BDBM50435122
(CHEMBL2391714)Show SMILES CC(C)[C@H](NC(=O)OC(C)(C)C)C(=O)NC(CCC(O)=O)P(=O)(Oc1ccccc1)Oc1ccccc1 |r| Show InChI InChI=1S/C26H35N2O8P/c1-18(2)23(28-25(32)34-26(3,4)5)24(31)27-21(16-17-22(29)30)37(33,35-19-12-8-6-9-13-19)36-20-14-10-7-11-15-20/h6-15,18,21,23H,16-17H2,1-5H3,(H,27,31)(H,28,32)(H,29,30)/t21?,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus endoproteinase GluC using Ac-Phe-Leu-Glu-ACC as substrate after 30 mins |
Bioorg Med Chem Lett 23: 1412-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.074
BindingDB Entry DOI: 10.7270/Q2SX6FKB |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50024592

((S)-1-Ammonium-2-phenyl-ethanephosphonic acid anio...)Show InChI InChI=1S/C8H12NO3P/c9-8(13(10,11)12)6-7-4-2-1-3-5-7/h1-5,8H,6,9H2,(H2,10,11,12)/t8-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of aminopeptidase N |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50316048

(1-amino-2-phenylethylphosphonic acid monophenyl es...)Show InChI InChI=1S/C14H16NO3P/c15-14(11-12-7-3-1-4-8-12)19(16,17)18-13-9-5-2-6-10-13/h1-10,14H,11,15H2,(H,16,17) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of aminopeptidase N |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50316047

(1-amino-2-methylbutylphosphonic acid monophenylest...)Show InChI InChI=1S/C11H18NO3P/c1-9(2)8-11(12)16(13,14)15-10-6-4-3-5-7-10/h3-7,9,11H,8,12H2,1-2H3,(H,13,14) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of aminopeptidase N |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50129679

((1-Amino-3-methyl-butyl)-phosphonic acid | 1-amino...)Show InChI InChI=1S/C5H14NO3P/c1-4(2)3-5(6)10(7,8)9/h4-5H,3,6H2,1-2H3,(H2,7,8,9) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of aminopeptidase N |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50184648
(CHEMBL378976 | [benzyloxycarbonylamino-(4-guanidin...)Show SMILES [#6]-[#16]-c1ccc(-[#8]P(=O)([#8]-c2ccc(-[#16]-[#6])cc2)[#6](-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C30H31N4O5PS2/c1-41-26-16-12-24(13-17-26)38-40(36,39-25-14-18-27(42-2)19-15-25)28(22-8-10-23(11-9-22)33-29(31)32)34-30(35)37-20-21-6-4-3-5-7-21/h3-19,28H,20H2,1-2H3,(H,34,35)(H4,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human uPA by chromogenic assay using Cbz-Val-Gly-Arg-pNA as chromogenic substrate |
Bioorg Med Chem Lett 16: 2886-90 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.002
BindingDB Entry DOI: 10.7270/Q22N51WR |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50184646
(CHEMBL379814 | [benzyloxycarbonylamino-(4-guanidin...)Show SMILES [#6]S(=O)(=O)c1ccc(-[#8]P(=O)([#8]-c2ccc(cc2)S([#6])(=O)=O)[#6](-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C30H31N4O9PS2/c1-45(37,38)26-16-12-24(13-17-26)42-44(36,43-25-14-18-27(19-15-25)46(2,39)40)28(22-8-10-23(11-9-22)33-29(31)32)34-30(35)41-20-21-6-4-3-5-7-21/h3-19,28H,20H2,1-2H3,(H,34,35)(H4,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human uPA by chromogenic assay using Cbz-Val-Gly-Arg-pNA as chromogenic substrate |
Bioorg Med Chem Lett 16: 2886-90 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.002
BindingDB Entry DOI: 10.7270/Q22N51WR |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50184644

(CHEMBL377857 | [benzyloxycarbonylamino-(4-guanidin...)Show SMILES [#6]C([#6])([#6])c1ccc(-[#8]P(=O)([#8]-c2ccc(cc2)C([#6])([#6])[#6])[#6](-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C36H43N4O5P/c1-35(2,3)27-14-20-30(21-15-27)44-46(42,45-31-22-16-28(17-23-31)36(4,5)6)32(26-12-18-29(19-13-26)39-33(37)38)40-34(41)43-24-25-10-8-7-9-11-25/h7-23,32H,24H2,1-6H3,(H,40,41)(H4,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human uPA by chromogenic assay using Cbz-Val-Gly-Arg-pNA as chromogenic substrate |
Bioorg Med Chem Lett 16: 2886-90 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.002
BindingDB Entry DOI: 10.7270/Q22N51WR |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50184647
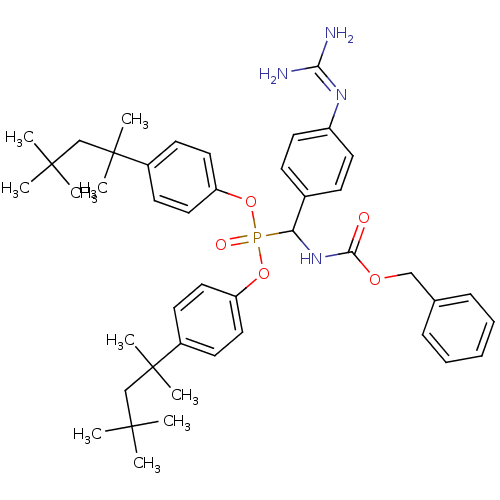
(CHEMBL378778 | [benzyloxycarbonylamino-(4-guanidin...)Show SMILES [#6]C([#6])([#6])[#6]C([#6])([#6])c1ccc(-[#8]P(=O)([#8]-c2ccc(cc2)C([#6])([#6])[#6]C([#6])([#6])[#6])[#6](-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C44H59N4O5P/c1-41(2,3)29-43(7,8)33-18-24-36(25-19-33)52-54(50,53-37-26-20-34(21-27-37)44(9,10)30-42(4,5)6)38(32-16-22-35(23-17-32)47-39(45)46)48-40(49)51-28-31-14-12-11-13-15-31/h11-27,38H,28-30H2,1-10H3,(H,48,49)(H4,45,46,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human uPA by chromogenic assay using Cbz-Val-Gly-Arg-pNA as chromogenic substrate |
Bioorg Med Chem Lett 16: 2886-90 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.002
BindingDB Entry DOI: 10.7270/Q22N51WR |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50184640
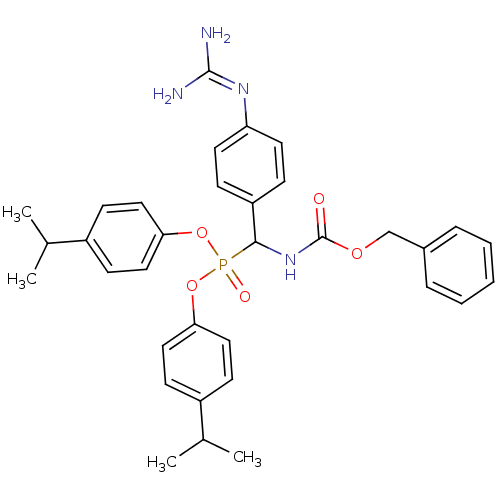
(CHEMBL378396 | [benzyloxycarbonylamino-(4-guanidin...)Show SMILES [#6]-[#6](-[#6])-c1ccc(-[#8]P(=O)([#8]-c2ccc(cc2)-[#6](-[#6])-[#6])[#6](-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C34H39N4O5P/c1-23(2)26-12-18-30(19-13-26)42-44(40,43-31-20-14-27(15-21-31)24(3)4)32(28-10-16-29(17-11-28)37-33(35)36)38-34(39)41-22-25-8-6-5-7-9-25/h5-21,23-24,32H,22H2,1-4H3,(H,38,39)(H4,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human uPA by chromogenic assay using Cbz-Val-Gly-Arg-pNA as chromogenic substrate |
Bioorg Med Chem Lett 16: 2886-90 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.002
BindingDB Entry DOI: 10.7270/Q22N51WR |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50204146

(1-amino-2-phenylethyl(1-hydroxy-3-phenylpropyl)pho...)Show InChI InChI=1S/C17H22NO3P/c18-16(13-15-9-5-2-6-10-15)22(20,21)17(19)12-11-14-7-3-1-4-8-14/h1-10,16,20-22H,11-13,18H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of APN |
Bioorg Med Chem Lett 17: 1516-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.028
BindingDB Entry DOI: 10.7270/Q2C24W3B |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50184645
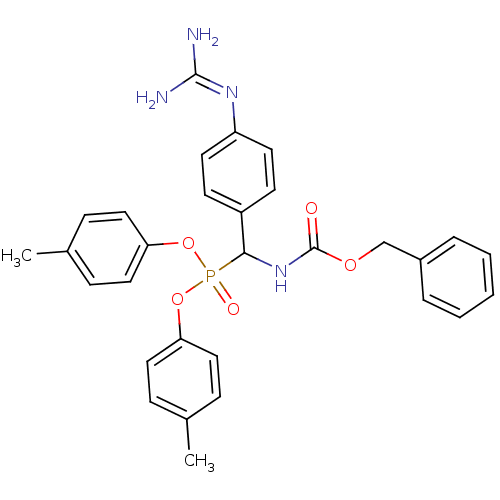
(CHEMBL209091 | [benzyloxycarbonylamino-(4-guanidin...)Show SMILES [#6]-c1ccc(-[#8]P(=O)([#8]-c2ccc(-[#6])cc2)[#6](-[#7]-[#6](=O)-[#8]-[#6]-c2ccccc2)-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C30H31N4O5P/c1-21-8-16-26(17-9-21)38-40(36,39-27-18-10-22(2)11-19-27)28(24-12-14-25(15-13-24)33-29(31)32)34-30(35)37-20-23-6-4-3-5-7-23/h3-19,28H,20H2,1-2H3,(H,34,35)(H4,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human uPA by chromogenic assay using Cbz-Val-Gly-Arg-pNA as chromogenic substrate |
Bioorg Med Chem Lett 16: 2886-90 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.002
BindingDB Entry DOI: 10.7270/Q22N51WR |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50244150
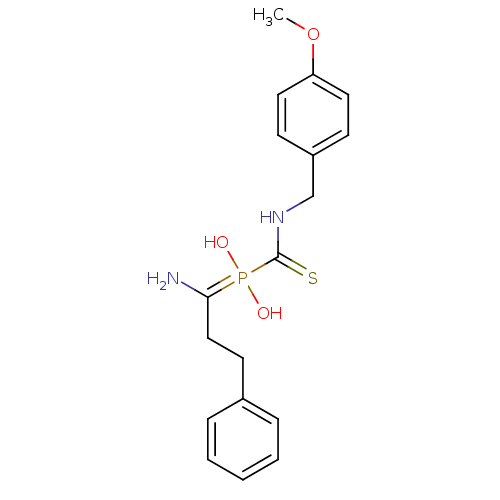
((4-methoxybenzyl)carbamothioyl(1-amino-3-phenylpro...)Show InChI InChI=1S/C18H23N2O3PS/c1-23-16-10-7-15(8-11-16)13-20-18(25)24(21,22)17(19)12-9-14-5-3-2-4-6-14/h2-8,10-11,21-22H,9,12-13,19H2,1H3,(H,20,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N |
Bioorg Med Chem Lett 18: 3734-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.050
BindingDB Entry DOI: 10.7270/Q2H41R6T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50184642
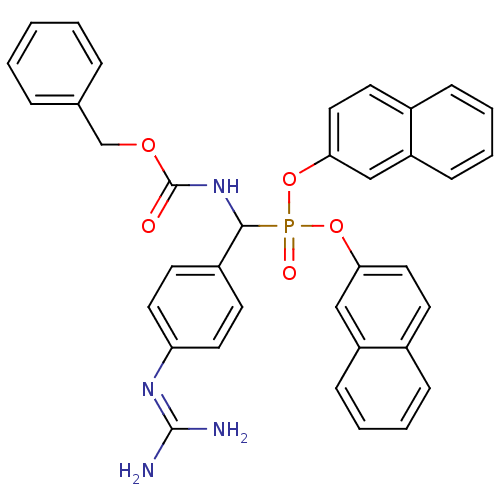
(CHEMBL378782 | [benzyloxycarbonylamino-(4-guanidin...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(cc1)-[#6](-[#7]-[#6](=O)-[#8]-[#6]-c1ccccc1)P(=O)([#8]-c1ccc2ccccc2c1)[#8]-c1ccc2ccccc2c1 Show InChI InChI=1S/C36H31N4O5P/c37-35(38)39-31-18-14-28(15-19-31)34(40-36(41)43-24-25-8-2-1-3-9-25)46(42,44-32-20-16-26-10-4-6-12-29(26)22-32)45-33-21-17-27-11-5-7-13-30(27)23-33/h1-23,34H,24H2,(H,40,41)(H4,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 455 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human uPA by chromogenic assay using Cbz-Val-Gly-Arg-pNA as chromogenic substrate |
Bioorg Med Chem Lett 16: 2886-90 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.002
BindingDB Entry DOI: 10.7270/Q22N51WR |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50316042

(1-amino-3-phenylpropylphosphonic acid mono-(4-isop...)Show InChI InChI=1S/C18H24NO3P/c1-14(2)16-9-11-17(12-10-16)22-23(20,21)18(19)13-8-15-6-4-3-5-7-15/h3-7,9-12,14,18H,8,13,19H2,1-2H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N using Leu-AMC as substrate |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50244148

(1-amino-3-methylbutyl(hexylcarbamothioyl)phosphini...)Show InChI InChI=1S/C12H27N2O2PS/c1-4-5-6-7-8-14-12(18)17(15,16)11(13)9-10(2)3/h10,15-16H,4-9,13H2,1-3H3,(H,14,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N |
Bioorg Med Chem Lett 18: 3734-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.050
BindingDB Entry DOI: 10.7270/Q2H41R6T |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50204160
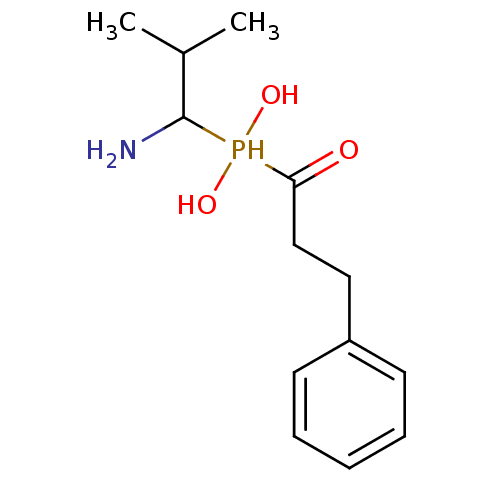
(1-amino-2-methylpropyl(1-hydroxy-3-phenylpropyl)ph...)Show InChI InChI=1S/C13H22NO3P/c1-10(2)13(14)18(16,17)12(15)9-8-11-6-4-3-5-7-11/h3-7,10,13,16-18H,8-9,14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of APN |
Bioorg Med Chem Lett 17: 1516-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.028
BindingDB Entry DOI: 10.7270/Q2C24W3B |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50204163
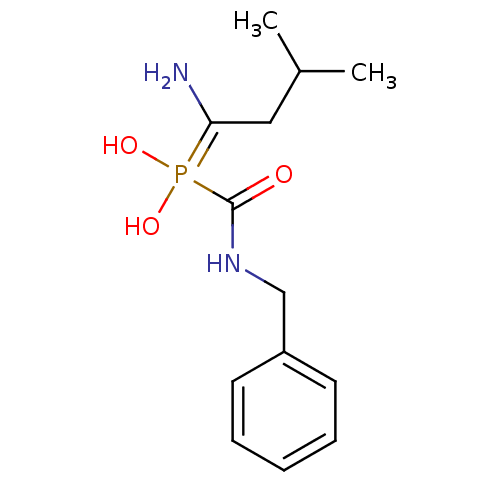
(CHEMBL227345 | alpha1-(1-amino-3-methylbutane)-alp...)Show InChI InChI=1S/C13H21N2O3P/c1-10(2)8-12(14)19(17,18)13(16)15-9-11-6-4-3-5-7-11/h3-7,10,17-18H,8-9,14H2,1-2H3,(H,15,16) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of APN |
Bioorg Med Chem Lett 17: 1516-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.028
BindingDB Entry DOI: 10.7270/Q2C24W3B |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50184643

(CHEMBL379807 | [benzyloxycarbonylamino-(4-guanidin...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ccc(cc1)-[#6](-[#7]-[#6](=O)-[#8]-[#6]-c1ccccc1)P(=O)([#8]-c1ccccc1)[#8]-c1ccccc1 Show InChI InChI=1S/C28H27N4O5P/c29-27(30)31-23-18-16-22(17-19-23)26(32-28(33)35-20-21-10-4-1-5-11-21)38(34,36-24-12-6-2-7-13-24)37-25-14-8-3-9-15-25/h1-19,26H,20H2,(H,32,33)(H4,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 532 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human uPA by chromogenic assay using Cbz-Val-Gly-Arg-pNA as chromogenic substrate |
Bioorg Med Chem Lett 16: 2886-90 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.002
BindingDB Entry DOI: 10.7270/Q22N51WR |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50204159
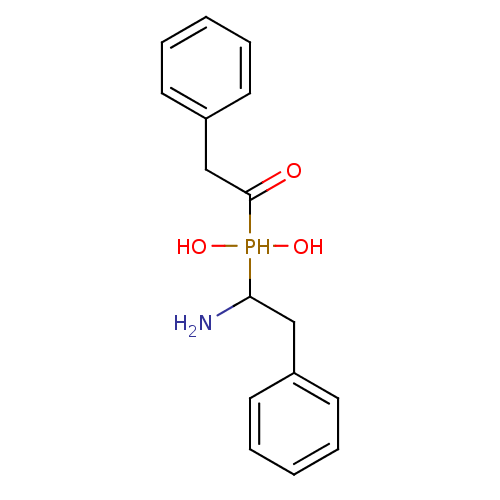
(CHEMBL226863 | alpha1-(1-amino-1-phenylmethane)-al...)Show InChI InChI=1S/C16H20NO3P/c17-15(11-13-7-3-1-4-8-13)21(19,20)16(18)12-14-9-5-2-6-10-14/h1-10,15,19-21H,11-12,17H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of APN |
Bioorg Med Chem Lett 17: 1516-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.028
BindingDB Entry DOI: 10.7270/Q2C24W3B |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50204161

(CHEMBL227290 | bis-alpha-(1-amino-1-phenylmethane)...)Show InChI InChI=1S/C16H21N2O2P/c17-15(11-13-7-3-1-4-8-13)21(19,20)16(18)12-14-9-5-2-6-10-14/h1-10,15,18-21H,11-12,17H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of APN |
Bioorg Med Chem Lett 17: 1516-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.028
BindingDB Entry DOI: 10.7270/Q2C24W3B |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50244108
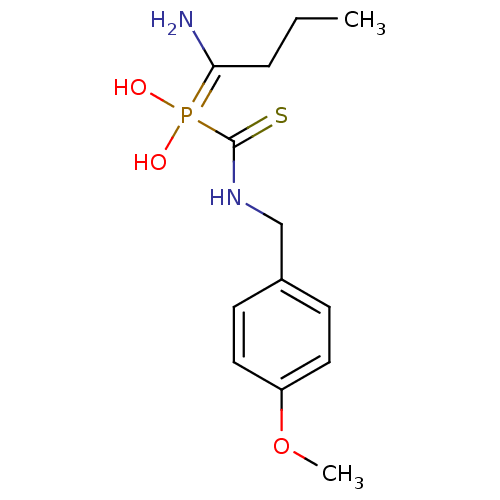
((4-methoxybenzyl)carbamothioyl(1-aminobutyl)phosph...)Show InChI InChI=1S/C13H21N2O3PS/c1-3-4-12(14)19(16,17)13(20)15-9-10-5-7-11(18-2)8-6-10/h5-8,16-17H,3-4,9,14H2,1-2H3,(H,15,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N |
Bioorg Med Chem Lett 18: 3734-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.050
BindingDB Entry DOI: 10.7270/Q2H41R6T |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50204157

(CHEMBL227340 | alpha1-(1-amino-1-phenylmethane)-al...)Show InChI InChI=1S/C17H23N2O2P/c18-16(12-11-14-7-3-1-4-8-14)22(20,21)17(19)13-15-9-5-2-6-10-15/h1-10,17-18,20-22H,11-13,19H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of APN |
Bioorg Med Chem Lett 17: 1516-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.028
BindingDB Entry DOI: 10.7270/Q2C24W3B |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50244146
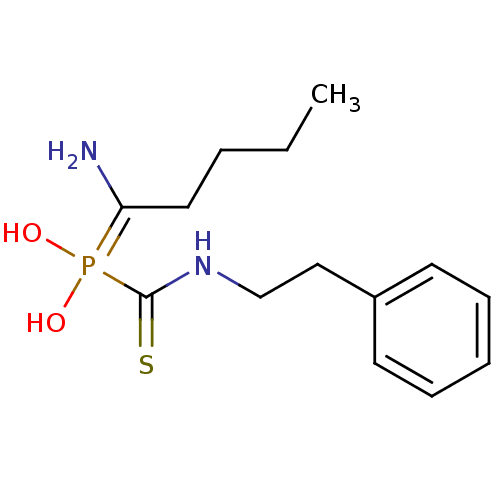
(1-aminopentyl(phenethylcarbamothioyl)phosphinic ac...)Show InChI InChI=1S/C14H23N2O2PS/c1-2-3-9-13(15)19(17,18)14(20)16-11-10-12-7-5-4-6-8-12/h4-8,17-18H,2-3,9-11,15H2,1H3,(H,16,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N |
Bioorg Med Chem Lett 18: 3734-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.050
BindingDB Entry DOI: 10.7270/Q2H41R6T |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50204149

(CHEMBL390323 | bis-alpha-(1-amino-3-phenylpropane)...)Show InChI InChI=1S/C18H25N2O2P/c19-17(13-11-15-7-3-1-4-8-15)23(21,22)18(20)14-12-16-9-5-2-6-10-16/h1-10,17,20-23H,11-14,19H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of APN |
Bioorg Med Chem Lett 17: 1516-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.028
BindingDB Entry DOI: 10.7270/Q2C24W3B |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50204148
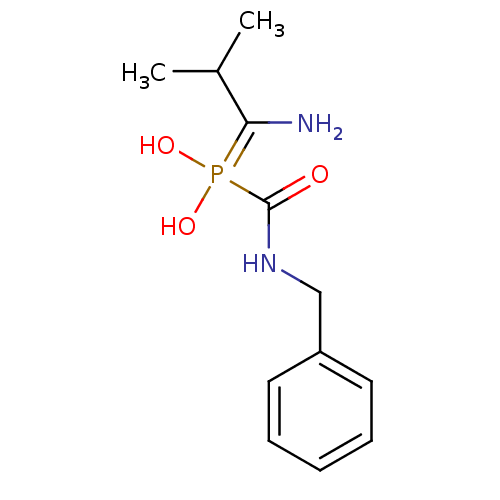
(CHEMBL227347 | alpha1-(1-amino-2-methylpropane)-al...)Show InChI InChI=1S/C12H19N2O3P/c1-9(2)11(13)18(16,17)12(15)14-8-10-6-4-3-5-7-10/h3-7,9,16-17H,8,13H2,1-2H3,(H,14,15) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of APN |
Bioorg Med Chem Lett 17: 1516-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.028
BindingDB Entry DOI: 10.7270/Q2C24W3B |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50204150
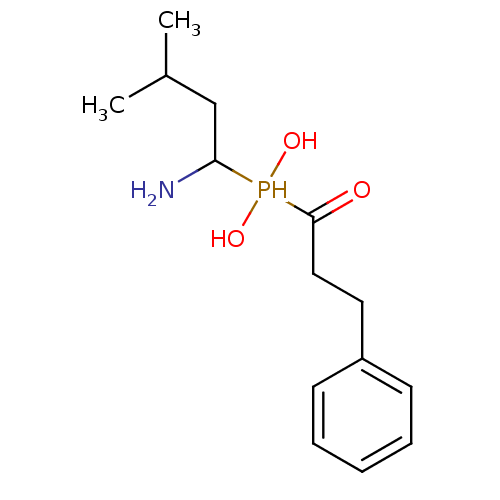
(1-amino-3-methylbutyl(1-hydroxy-3-phenylpropyl)pho...)Show InChI InChI=1S/C14H24NO3P/c1-11(2)10-13(15)19(17,18)14(16)9-8-12-6-4-3-5-7-12/h3-7,11,13,17-19H,8-10,15H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of APN |
Bioorg Med Chem Lett 17: 1516-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.028
BindingDB Entry DOI: 10.7270/Q2C24W3B |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50316033
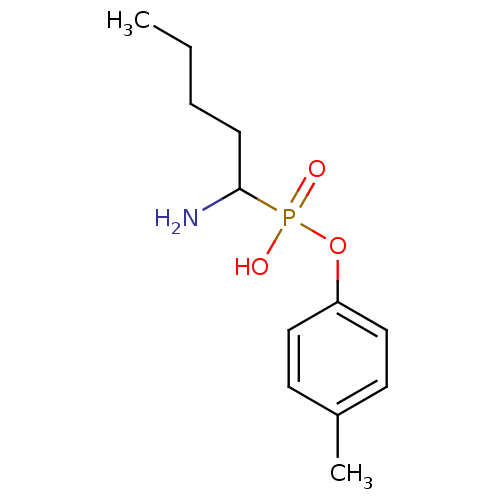
(1-aminopentylphosphonic acid mono-(4-methylphenyl)...)Show InChI InChI=1S/C12H20NO3P/c1-3-4-5-12(13)17(14,15)16-11-8-6-10(2)7-9-11/h6-9,12H,3-5,13H2,1-2H3,(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N using Leu-AMC as substrate |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50316028

(1-aminopropylphosphonic acid mono-(4-tbutylphenyl)...)Show InChI InChI=1S/C13H22NO3P/c1-5-12(14)18(15,16)17-11-8-6-10(7-9-11)13(2,3)4/h6-9,12H,5,14H2,1-4H3,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N using Leu-AMC as substrate |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50244106

((4-methoxybenzyl)carbamothioyl(1-aminoethyl)phosph...)Show InChI InChI=1S/C11H17N2O3PS/c1-8(12)17(14,15)11(18)13-7-9-3-5-10(16-2)6-4-9/h3-6,14-15H,7,12H2,1-2H3,(H,13,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N |
Bioorg Med Chem Lett 18: 3734-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.050
BindingDB Entry DOI: 10.7270/Q2H41R6T |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50244110
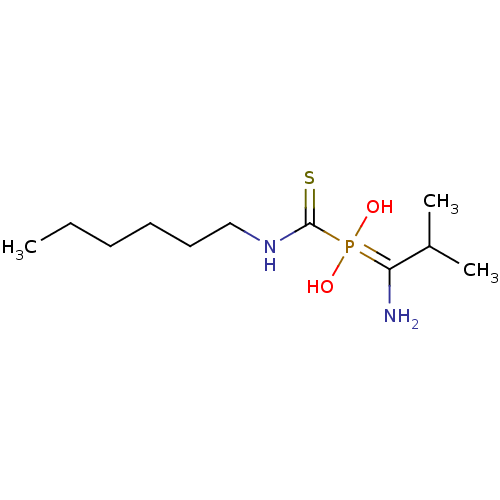
(1-amino-2-methylpropyl(hexylcarbamothioyl)phosphin...)Show InChI InChI=1S/C11H25N2O2PS/c1-4-5-6-7-8-13-11(17)16(14,15)10(12)9(2)3/h9,14-15H,4-8,12H2,1-3H3,(H,13,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N |
Bioorg Med Chem Lett 18: 3734-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.050
BindingDB Entry DOI: 10.7270/Q2H41R6T |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50316034
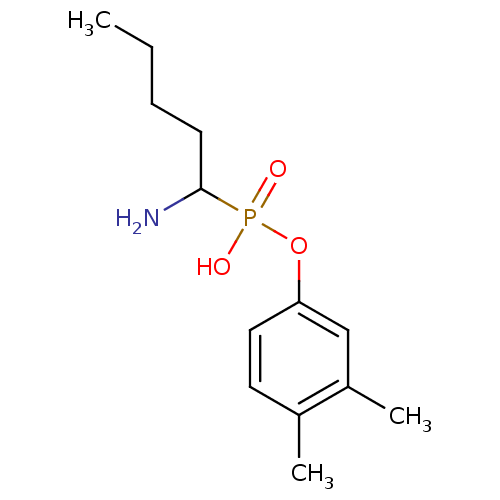
(1-aminopentylphosphonic acid mono-(3,4-dimethylphe...)Show InChI InChI=1S/C13H22NO3P/c1-4-5-6-13(14)18(15,16)17-12-8-7-10(2)11(3)9-12/h7-9,13H,4-6,14H2,1-3H3,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N using Leu-AMC as substrate |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50244109
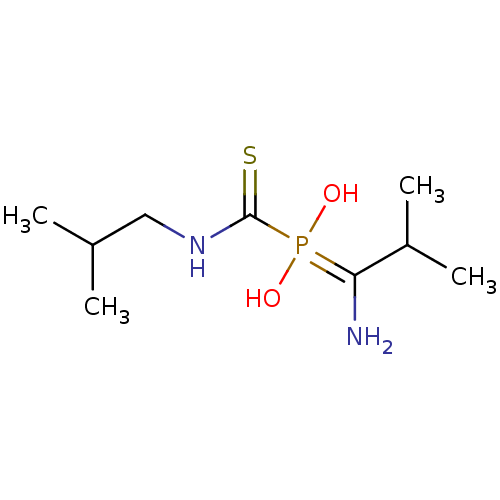
(1-amino-2-methylpropyl(isobutylcarbamothioyl)phosp...)Show InChI InChI=1S/C9H21N2O2PS/c1-6(2)5-11-9(15)14(12,13)8(10)7(3)4/h6-7,12-13H,5,10H2,1-4H3,(H,11,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N |
Bioorg Med Chem Lett 18: 3734-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.050
BindingDB Entry DOI: 10.7270/Q2H41R6T |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50316043

(1-amino-3-phenylpropylphosphonic acid mono-(4-t-bu...)Show InChI InChI=1S/C19H26NO3P/c1-19(2,3)16-10-12-17(13-11-16)23-24(21,22)18(20)14-9-15-7-5-4-6-8-15/h4-8,10-13,18H,9,14,20H2,1-3H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N using Leu-AMC as substrate |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N |
Bioorg Med Chem Lett 18: 3734-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.050
BindingDB Entry DOI: 10.7270/Q2H41R6T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney aminopeptidase N using Leu-AMC as substrate |
Bioorg Med Chem 18: 2930-6 (2010)
Article DOI: 10.1016/j.bmc.2010.02.056
BindingDB Entry DOI: 10.7270/Q2FN16C0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data