

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019696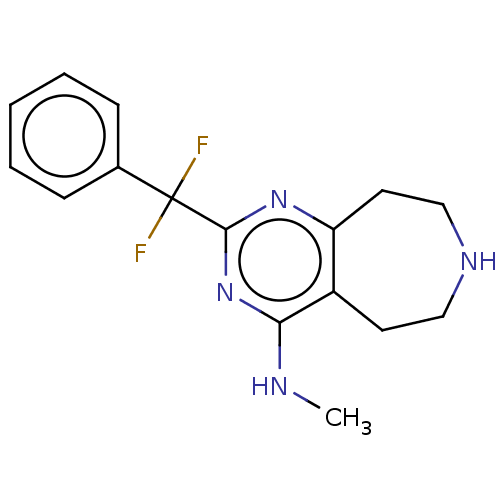 (CHEMBL3286557) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4814 (CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz-Zentrum Dresden-Rossendorf eV Curated by ChEMBL | Assay Description Competitive inhibition of VEGFR2 by qPCR method | Bioorg Med Chem Lett 22: 2850-5 (2012) Article DOI: 10.1016/j.bmcl.2012.02.068 BindingDB Entry DOI: 10.7270/Q2CZ3878 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019685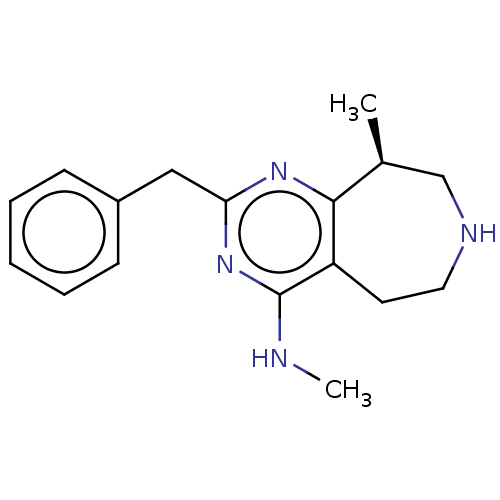 (CHEMBL3286556) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition constant using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substra... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019682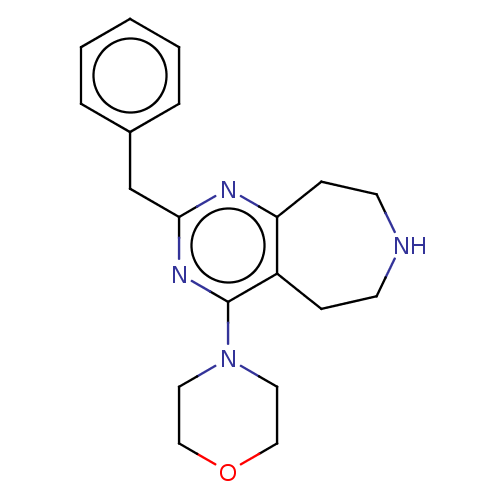 (CHEMBL3286564) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019664 (CHEMBL3286562) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019691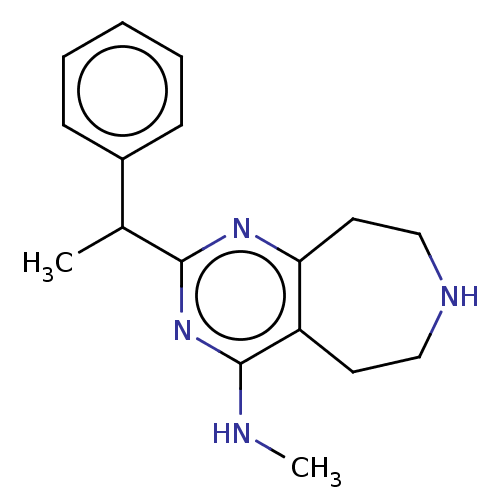 (CHEMBL3286565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019663 (CHEMBL3286561) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019662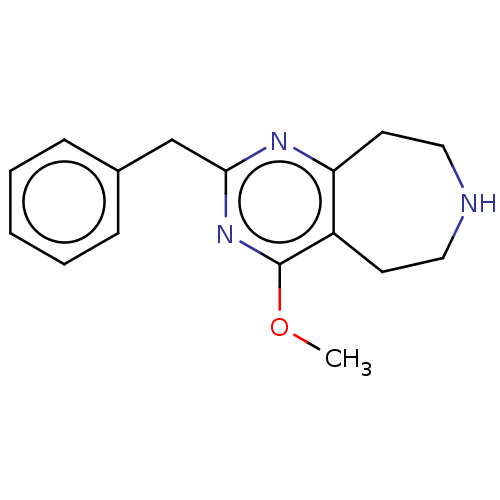 (CHEMBL3286560) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019691 (CHEMBL3286565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019692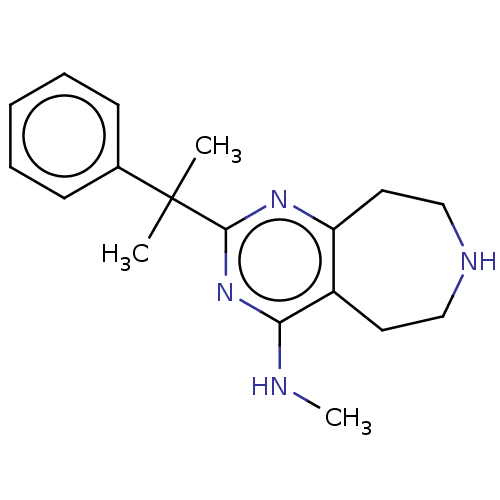 (CHEMBL3286566) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019695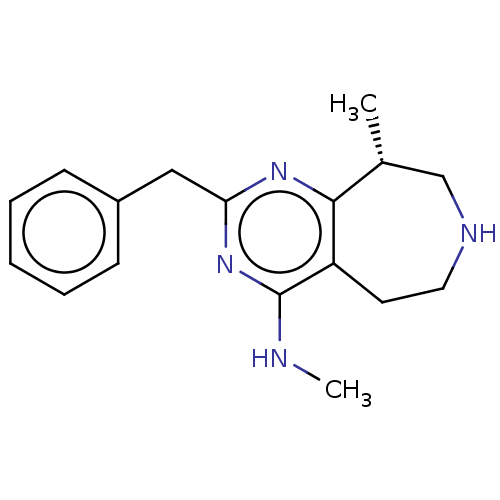 (CHEMBL3286555) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019694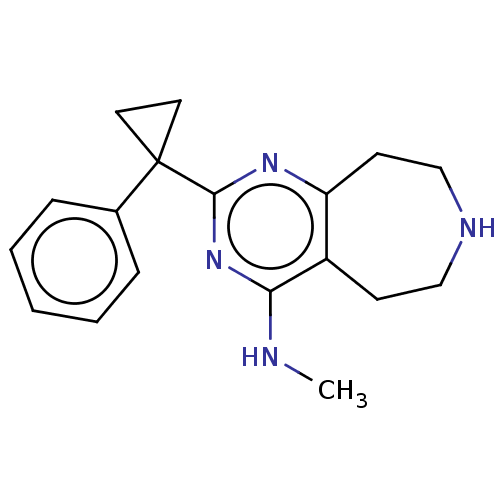 (CHEMBL3286554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50342538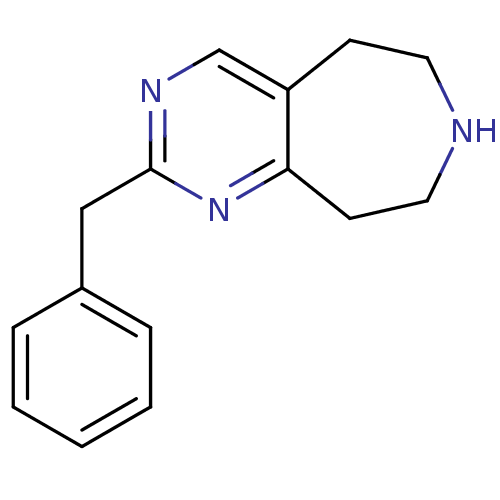 (2-benzyl-6,7,8,9-tetrahydro-5H-pyrimido[5,4-d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019674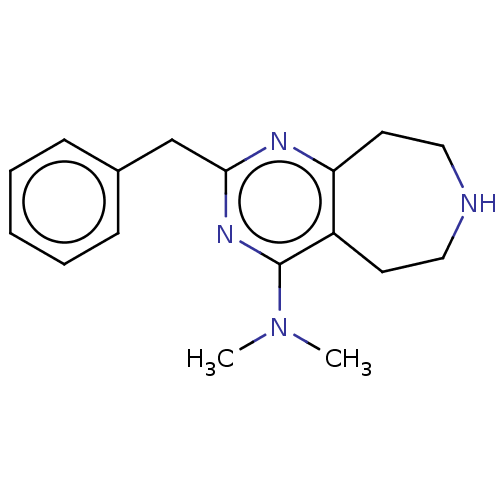 (CHEMBL3286563) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 233 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50019661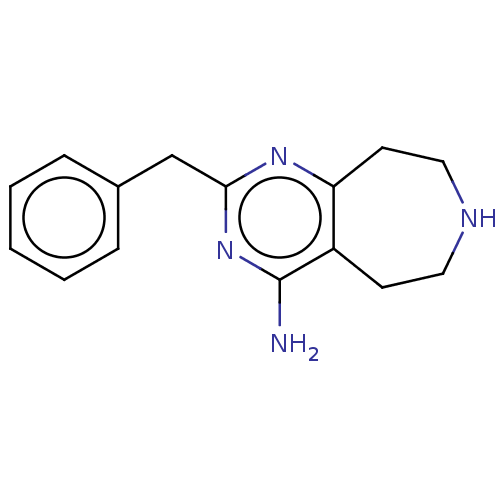 (CHEMBL3286559) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 958 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay | J Med Chem 57: 5258-69 (2014) Article DOI: 10.1021/jm5003292 BindingDB Entry DOI: 10.7270/Q2J104RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292422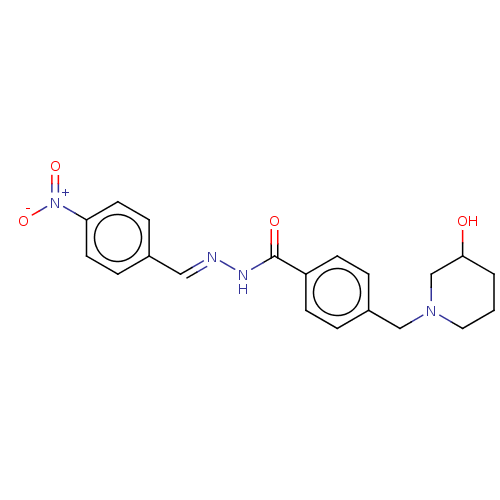 (CHEMBL4175724) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292422 (CHEMBL4175724) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50524422 (CHEMBL4557635) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition constant using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substra... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292419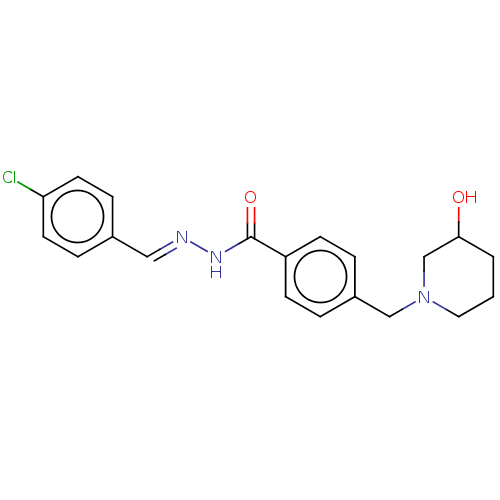 (CHEMBL4163724) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292419 (CHEMBL4163724) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292536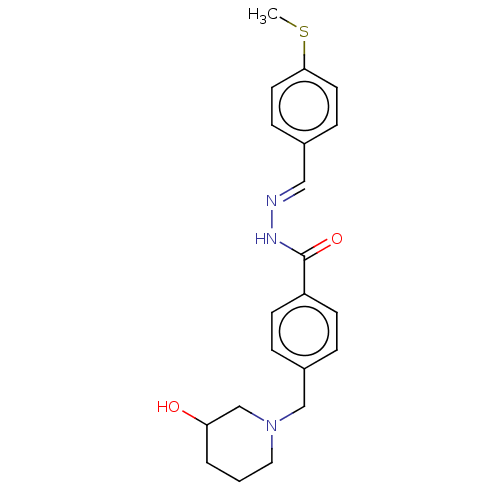 (CHEMBL4170841) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Apparent affinity to inhibit binding of [3H]-pCCK-8 to Cholecystokinin type A receptor of guinea pig pancreatic membranes | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292536 (CHEMBL4170841) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Apparent affinity to inhibit binding of [3H]-pCCK-8 to Cholecystokinin type A receptor of guinea pig pancreatic membranes | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292510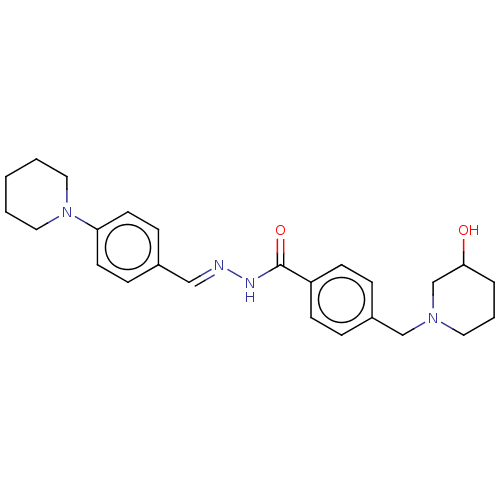 (CHEMBL4172314) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Affinity of compound to Sigma opioid receptor labelled with [3H](+)-3-PPP | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292510 (CHEMBL4172314) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Affinity of compound to Sigma opioid receptor labelled with [3H](+)-3-PPP | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50524423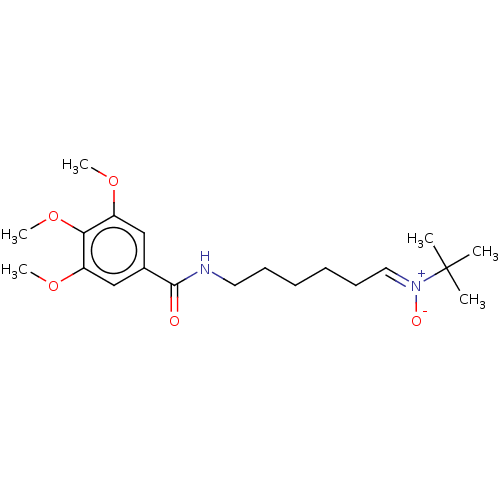 (CHEMBL4456422) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition constant using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substra... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292423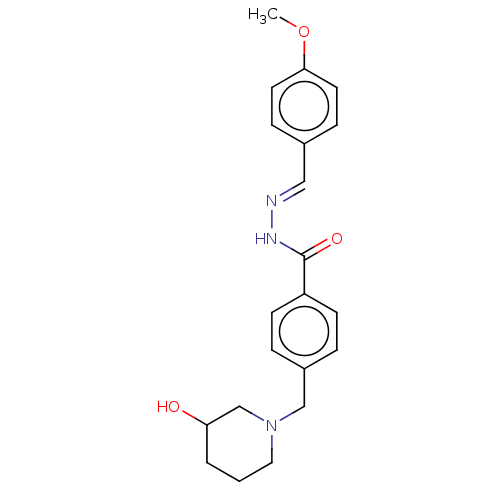 (CHEMBL4167867) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292423 (CHEMBL4167867) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292442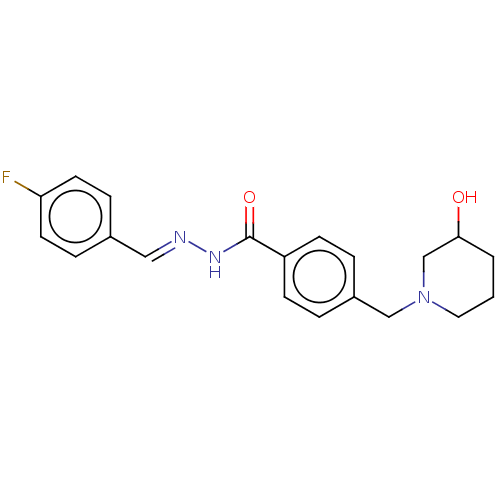 (CHEMBL4171226) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292442 (CHEMBL4171226) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292526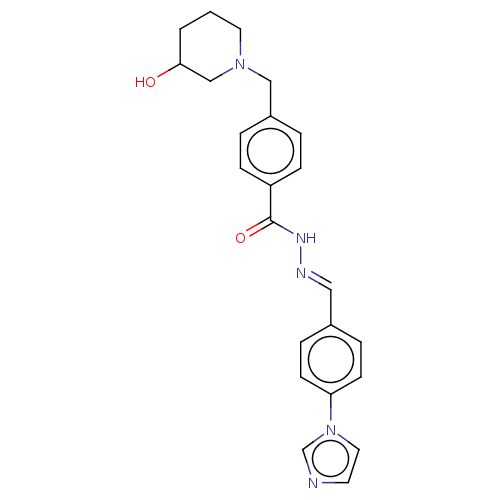 (CHEMBL4164436) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292526 (CHEMBL4164436) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50514163 (CHEMBL4568985) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... | J Med Chem 62: 10645-10663 (2019) Article DOI: 10.1021/acs.jmedchem.9b00966 BindingDB Entry DOI: 10.7270/Q27084RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative glyoxylate/hydroxypyruvate reductase B (Staphylococcus aureus (strain SA113)) | BDBM392802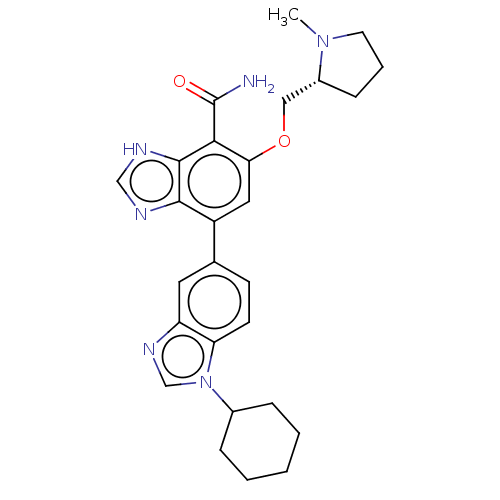 (7-(1-cyclohexylbenzimidazol-5-yl)-5-[[(2R)-1-methy...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The assay buffer “AB” contained 50 mM Hepes pH8.0, 10 mM MgCl2, 50 mM KCl, 0.012% Triton-X100, 10 mM DTT and 100 nM Myelin-basic protein. The followi... | Bioorg Med Chem Lett 18: 5018-22 (2008) BindingDB Entry DOI: 10.7270/Q2R213Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50514163 (CHEMBL4568985) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... | J Med Chem 62: 10645-10663 (2019) Article DOI: 10.1021/acs.jmedchem.9b00966 BindingDB Entry DOI: 10.7270/Q27084RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50514158 (CHEMBL4526913) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... | J Med Chem 62: 10645-10663 (2019) Article DOI: 10.1021/acs.jmedchem.9b00966 BindingDB Entry DOI: 10.7270/Q27084RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative glyoxylate/hydroxypyruvate reductase B (Staphylococcus aureus (strain SA113)) | BDBM392796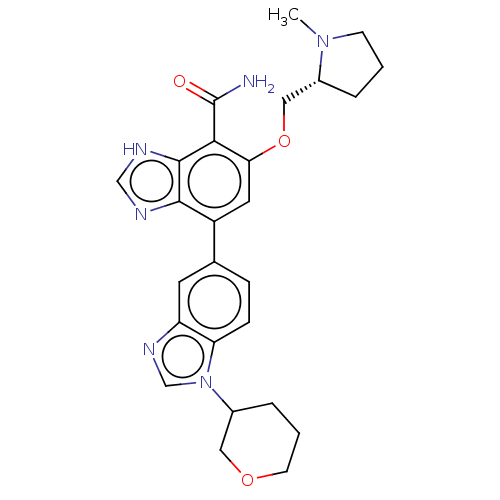 (5-[[(2R)-1-methylpyrrolidin-2-yl]methoxy]-7-(1-tet...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The assay buffer “AB” contained 50 mM Hepes pH8.0, 10 mM MgCl2, 50 mM KCl, 0.012% Triton-X100, 10 mM DTT and 100 nM Myelin-basic protein. The followi... | Bioorg Med Chem Lett 18: 5018-22 (2008) BindingDB Entry DOI: 10.7270/Q2R213Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanine--D-alanyl carrier protein ligase (Enterococcus faecalis (strain ATCC 700802 / V583)) | BDBM392796 (5-[[(2R)-1-methylpyrrolidin-2-yl]methoxy]-7-(1-tet...) | MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The assay buffer “AB” contained 50 mM Hepes pH8.0, 10 mM MgCl2, 50 mM KCl, 0.012% Triton-X100, 10 mM DTT and 100 nM Myelin-basic protein. The followi... | Bioorg Med Chem Lett 18: 5018-22 (2008) BindingDB Entry DOI: 10.7270/Q2R213Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanine--D-alanyl carrier protein ligase (Enterococcus faecalis (strain ATCC 700802 / V583)) | BDBM392802 (7-(1-cyclohexylbenzimidazol-5-yl)-5-[[(2R)-1-methy...) | MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The assay buffer “AB” contained 50 mM Hepes pH8.0, 10 mM MgCl2, 50 mM KCl, 0.012% Triton-X100, 10 mM DTT and 100 nM Myelin-basic protein. The followi... | Bioorg Med Chem Lett 18: 5018-22 (2008) BindingDB Entry DOI: 10.7270/Q2R213Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative glyoxylate/hydroxypyruvate reductase B (Staphylococcus aureus (strain SA113)) | BDBM392744 (7-(1-Cyclohexyl-1H-benzimidazol-5-yl)-5-(hexahydro...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The assay buffer “AB” contained 50 mM Hepes pH8.0, 10 mM MgCl2, 50 mM KCl, 0.012% Triton-X100, 10 mM DTT and 100 nM Myelin-basic protein. The followi... | Bioorg Med Chem Lett 18: 5018-22 (2008) BindingDB Entry DOI: 10.7270/Q2R213Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50514163 (CHEMBL4568985) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... | J Med Chem 62: 10645-10663 (2019) Article DOI: 10.1021/acs.jmedchem.9b00966 BindingDB Entry DOI: 10.7270/Q27084RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanine--D-alanyl carrier protein ligase (Enterococcus faecium E1679) | BDBM392794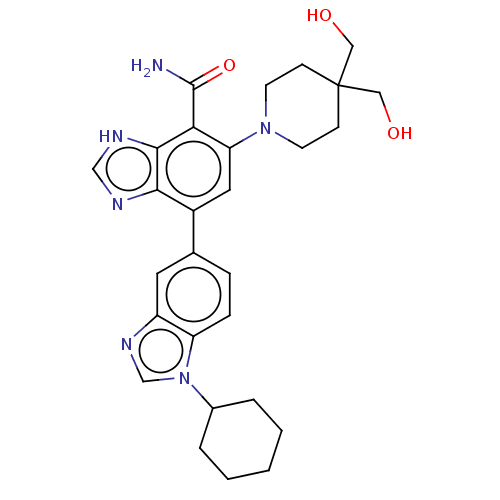 (5-[4,4-bis(hydroxymethyl)-1-piperidyl]-7-(1-cycloh...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The assay buffer “AB” contained 50 mM Hepes pH8.0, 10 mM MgCl2, 50 mM KCl, 0.012% Triton-X100, 10 mM DTT and 100 nM Myelin-basic protein. The followi... | Bioorg Med Chem Lett 18: 5018-22 (2008) BindingDB Entry DOI: 10.7270/Q2R213Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50514161 (CHEMBL4469705) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... | J Med Chem 62: 10645-10663 (2019) Article DOI: 10.1021/acs.jmedchem.9b00966 BindingDB Entry DOI: 10.7270/Q27084RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanine--D-alanyl carrier protein ligase (Enterococcus faecalis (strain ATCC 700802 / V583)) | BDBM392769 (1′-Cyclohexyl-6-[methyl-(2-pyrrolidin-1-yl-e...) | MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The assay buffer “AB” contained 50 mM Hepes pH8.0, 10 mM MgCl2, 50 mM KCl, 0.012% Triton-X100, 10 mM DTT and 100 nM Myelin-basic protein. The followi... | Bioorg Med Chem Lett 18: 5018-22 (2008) BindingDB Entry DOI: 10.7270/Q2R213Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50514158 (CHEMBL4526913) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... | J Med Chem 62: 10645-10663 (2019) Article DOI: 10.1021/acs.jmedchem.9b00966 BindingDB Entry DOI: 10.7270/Q27084RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151638 (8''-chloro-5''-(5-hydroxy-1,2,4-oxadiazol-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4627-31 (2004) Article DOI: 10.1016/j.bmcl.2004.07.010 BindingDB Entry DOI: 10.7270/Q2TT4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-alanine--D-alanyl carrier protein ligase (Enterococcus faecium E1679) | BDBM392802 (7-(1-cyclohexylbenzimidazol-5-yl)-5-[[(2R)-1-methy...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The assay buffer “AB” contained 50 mM Hepes pH8.0, 10 mM MgCl2, 50 mM KCl, 0.012% Triton-X100, 10 mM DTT and 100 nM Myelin-basic protein. The followi... | Bioorg Med Chem Lett 18: 5018-22 (2008) BindingDB Entry DOI: 10.7270/Q2R213Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151562 (7-{5-[(Z)-Cyclohexylimino]-4-methyl-4,5-dihydro-[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4607-13 (2004) Article DOI: 10.1016/j.bmcl.2004.07.008 BindingDB Entry DOI: 10.7270/Q2377855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151635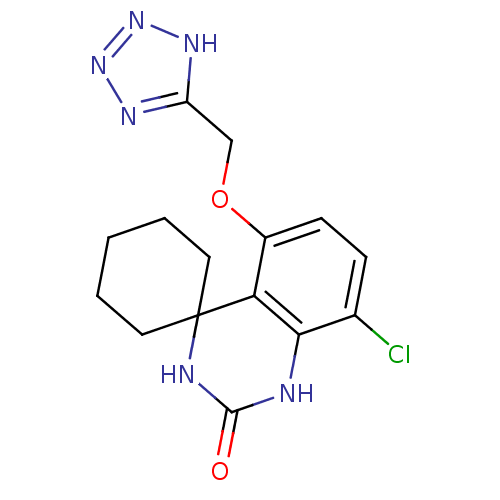 (8''-chloro-5''-(1H-1,2,3,4-tetraazol-5-ylmethoxy)s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4627-31 (2004) Article DOI: 10.1016/j.bmcl.2004.07.010 BindingDB Entry DOI: 10.7270/Q2TT4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50514160 (CHEMBL4557604) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... | J Med Chem 62: 10645-10663 (2019) Article DOI: 10.1021/acs.jmedchem.9b00966 BindingDB Entry DOI: 10.7270/Q27084RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 996 total ) | Next | Last >> |