 Found 82 hits with Last Name = 'osheroff' and Initial = 'n'
Found 82 hits with Last Name = 'osheroff' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597266
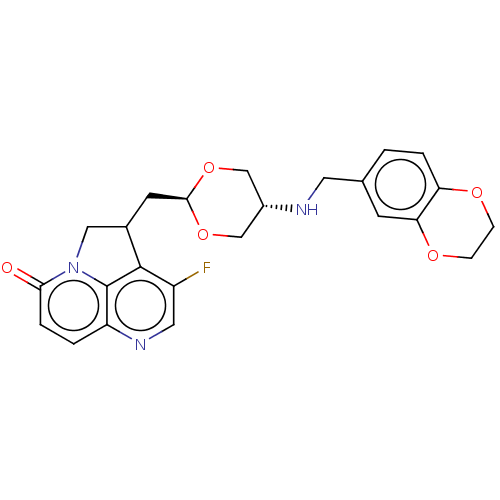
(CHEMBL5200272)Show SMILES Fc1cnc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4ccc5OCCOc5c4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.84,;-3.03,-2.61,;-3.03,-4.15,;-4.37,-4.92,;-5.7,-4.15,;-7.03,-4.92,;-8.37,-4.15,;-8.37,-2.61,;-9.7,-1.84,;-7.03,-1.84,;-6.47,-.41,;-4.93,-.41,;-4.16,.92,;-2.62,.92,;-1.85,2.26,;-.31,2.26,;.46,.92,;-.31,-.41,;-1.85,-.41,;2,.92,;2.77,2.26,;4.31,2.26,;5.08,.92,;6.62,.92,;7.39,2.26,;8.93,2.26,;9.7,3.59,;8.93,4.92,;7.39,4.92,;6.62,3.59,;5.08,3.59,;-4.37,-1.84,;-5.7,-2.61,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human COX1 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00774
BindingDB Entry DOI: 10.7270/Q26H4N2X |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597278
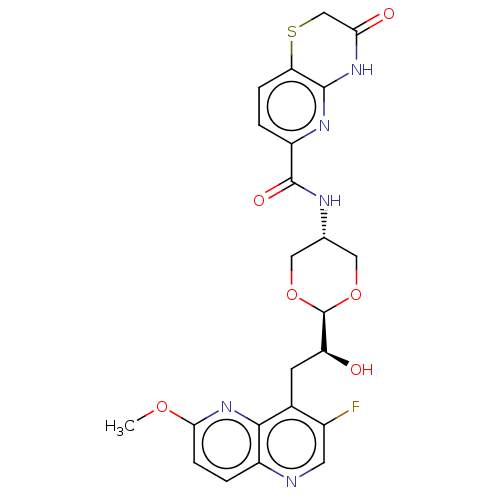
(CHEMBL5196889)Show SMILES [H][C@@]1(OC[C@@H](CO1)NC(=O)c1ccc2SCC(=O)Nc2n1)[C@@H](O)Cc1c(F)cnc2ccc(OC)nc12 |r,wD:21.24,4.7,1.0,(-1.88,-1.31,;-1.49,.18,;-.73,1.51,;.81,1.5,;1.57,.17,;.8,-1.15,;-.72,-1.14,;3.11,.17,;3.88,1.5,;3.11,2.83,;5.42,1.5,;6.18,.16,;7.72,.15,;8.49,1.48,;10.02,1.47,;10.8,2.8,;10.04,4.14,;10.81,5.48,;8.49,4.15,;7.72,2.82,;6.18,2.82,;-3.01,.18,;-3.34,1.68,;-4.16,-.85,;-4.15,-2.39,;-2.82,-3.15,;-1.5,-2.37,;-2.81,-4.7,;-4.14,-5.47,;-5.47,-4.7,;-6.81,-5.48,;-8.14,-4.71,;-8.14,-3.16,;-9.48,-2.4,;-10.81,-3.17,;-6.81,-2.4,;-5.47,-3.16,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597279
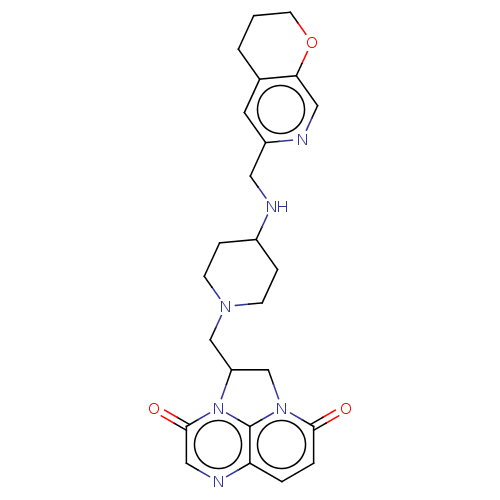
(CHEMBL4537408)Show SMILES O=c1ccc2ncc(=O)n3C(CN4CCC(CC4)NCc4cc5CCCOc5cn4)Cn1c23 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597276
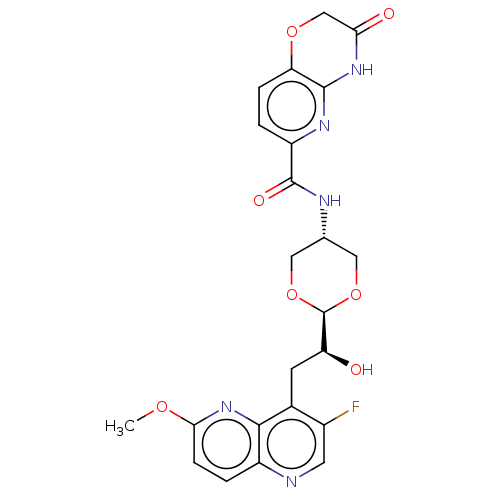
(CHEMBL5177294)Show SMILES [H][C@@]1(OC[C@@H](CO1)NC(=O)c1ccc2OCC(=O)Nc2n1)[C@@H](O)Cc1c(F)cnc2ccc(OC)nc12 |r,wD:21.24,4.7,1.0,(-1.88,-1.31,;-1.49,.18,;-.73,1.51,;.81,1.5,;1.57,.17,;.8,-1.15,;-.72,-1.14,;3.11,.17,;3.88,1.5,;3.11,2.83,;5.42,1.5,;6.18,.16,;7.71,.15,;8.49,1.48,;10.02,1.47,;10.8,2.8,;10.04,4.14,;10.81,5.48,;8.49,4.15,;7.71,2.82,;6.18,2.82,;-3.01,.18,;-3.34,1.68,;-4.16,-.85,;-4.15,-2.39,;-2.82,-3.15,;-1.5,-2.37,;-2.81,-4.7,;-4.14,-5.47,;-5.47,-4.7,;-6.81,-5.48,;-8.14,-4.71,;-8.14,-3.16,;-9.48,-2.4,;-10.81,-3.17,;-6.81,-2.4,;-5.47,-3.16,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597268
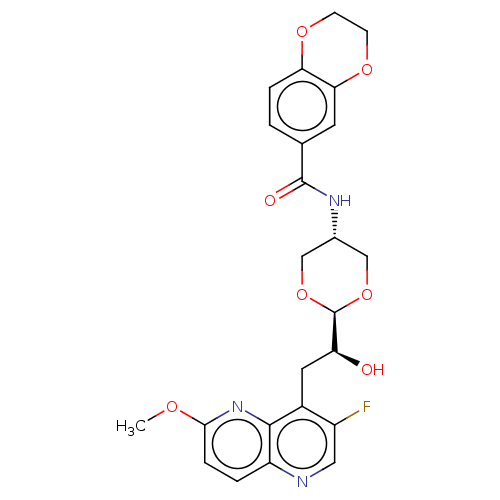
(CHEMBL5171625)Show SMILES [H][C@@]1(OC[C@@H](CO1)NC(=O)c1ccc2OCCOc2c1)[C@@H](O)Cc1c(F)cnc2ccc(OC)nc12 |r,wD:20.23,4.7,1.0,(-1.88,-.64,;-1.49,.84,;-.73,2.18,;.81,2.16,;1.57,.84,;.8,-.49,;-.72,-.48,;3.11,.83,;3.89,2.16,;3.12,3.5,;5.43,2.16,;6.19,.82,;7.72,.81,;8.5,2.15,;10.03,2.14,;10.81,3.47,;10.05,4.81,;8.5,4.82,;7.72,3.49,;6.19,3.49,;-3.01,.84,;-3.34,2.35,;-4.16,-.19,;-4.15,-1.73,;-2.82,-2.49,;-1.49,-1.71,;-2.81,-4.04,;-4.14,-4.81,;-5.48,-4.04,;-6.81,-4.82,;-8.15,-4.05,;-8.14,-2.5,;-9.48,-1.74,;-10.81,-2.51,;-6.81,-1.74,;-5.48,-2.5,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597267
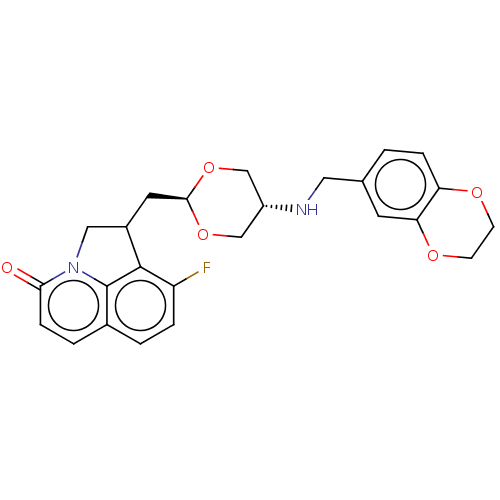
(CHEMBL5201666)Show SMILES Fc1ccc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4ccc5OCCOc5c4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.84,;-3.03,-2.61,;-3.03,-4.15,;-4.37,-4.92,;-5.7,-4.15,;-7.03,-4.92,;-8.37,-4.15,;-8.37,-2.61,;-9.7,-1.84,;-7.03,-1.84,;-6.47,-.41,;-4.93,-.41,;-4.16,.92,;-2.62,.92,;-1.85,2.26,;-.31,2.26,;.46,.92,;-.31,-.41,;-1.85,-.41,;2,.92,;2.77,2.26,;4.31,2.26,;5.08,.92,;6.62,.92,;7.39,2.26,;8.93,2.26,;9.7,3.59,;8.93,4.92,;7.39,4.92,;6.62,3.59,;5.08,3.59,;-4.37,-1.84,;-5.7,-2.61,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597278

(CHEMBL5196889)Show SMILES [H][C@@]1(OC[C@@H](CO1)NC(=O)c1ccc2SCC(=O)Nc2n1)[C@@H](O)Cc1c(F)cnc2ccc(OC)nc12 |r,wD:21.24,4.7,1.0,(-1.88,-1.31,;-1.49,.18,;-.73,1.51,;.81,1.5,;1.57,.17,;.8,-1.15,;-.72,-1.14,;3.11,.17,;3.88,1.5,;3.11,2.83,;5.42,1.5,;6.18,.16,;7.72,.15,;8.49,1.48,;10.02,1.47,;10.8,2.8,;10.04,4.14,;10.81,5.48,;8.49,4.15,;7.72,2.82,;6.18,2.82,;-3.01,.18,;-3.34,1.68,;-4.16,-.85,;-4.15,-2.39,;-2.82,-3.15,;-1.5,-2.37,;-2.81,-4.7,;-4.14,-5.47,;-5.47,-4.7,;-6.81,-5.48,;-8.14,-4.71,;-8.14,-3.16,;-9.48,-2.4,;-10.81,-3.17,;-6.81,-2.4,;-5.47,-3.16,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597266

(CHEMBL5200272)Show SMILES Fc1cnc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4ccc5OCCOc5c4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.84,;-3.03,-2.61,;-3.03,-4.15,;-4.37,-4.92,;-5.7,-4.15,;-7.03,-4.92,;-8.37,-4.15,;-8.37,-2.61,;-9.7,-1.84,;-7.03,-1.84,;-6.47,-.41,;-4.93,-.41,;-4.16,.92,;-2.62,.92,;-1.85,2.26,;-.31,2.26,;.46,.92,;-.31,-.41,;-1.85,-.41,;2,.92,;2.77,2.26,;4.31,2.26,;5.08,.92,;6.62,.92,;7.39,2.26,;8.93,2.26,;9.7,3.59,;8.93,4.92,;7.39,4.92,;6.62,3.59,;5.08,3.59,;-4.37,-1.84,;-5.7,-2.61,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597270
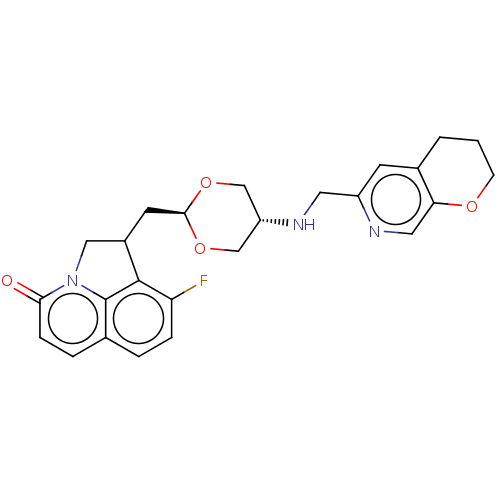
(CHEMBL5195074)Show SMILES Fc1ccc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4cc5CCCOc5cn4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.85,;-3.03,-2.62,;-3.03,-4.16,;-4.37,-4.93,;-5.7,-4.16,;-7.03,-4.93,;-8.37,-4.16,;-8.37,-2.62,;-9.7,-1.85,;-7.03,-1.85,;-6.47,-.41,;-4.93,-.41,;-4.16,.91,;-2.62,.91,;-1.85,2.25,;-.31,2.25,;.46,.91,;-.31,-.41,;-1.85,-.41,;2,.91,;2.77,2.25,;4.31,2.25,;5.08,3.59,;6.62,3.59,;7.38,4.93,;8.93,4.92,;9.7,3.58,;8.92,2.25,;7.39,2.25,;6.62,.91,;5.08,.91,;-4.37,-1.85,;-5.7,-2.62,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597267

(CHEMBL5201666)Show SMILES Fc1ccc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4ccc5OCCOc5c4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.84,;-3.03,-2.61,;-3.03,-4.15,;-4.37,-4.92,;-5.7,-4.15,;-7.03,-4.92,;-8.37,-4.15,;-8.37,-2.61,;-9.7,-1.84,;-7.03,-1.84,;-6.47,-.41,;-4.93,-.41,;-4.16,.92,;-2.62,.92,;-1.85,2.26,;-.31,2.26,;.46,.92,;-.31,-.41,;-1.85,-.41,;2,.92,;2.77,2.26,;4.31,2.26,;5.08,.92,;6.62,.92,;7.39,2.26,;8.93,2.26,;9.7,3.59,;8.93,4.92,;7.39,4.92,;6.62,3.59,;5.08,3.59,;-4.37,-1.84,;-5.7,-2.61,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50261767
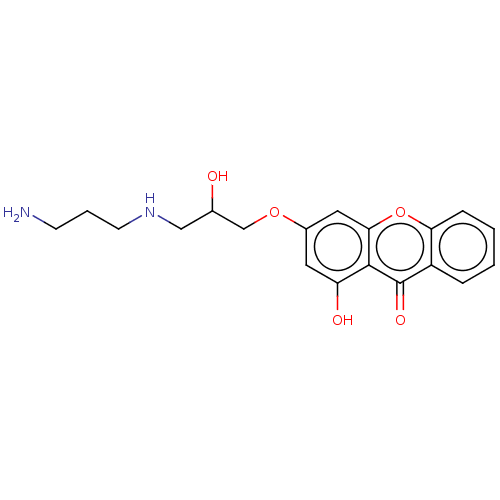
(CHEMBL4061116)Show SMILES Cl.Cl.NCCCNCC(O)COc1cc(O)c2c(c1)oc1ccccc1c2=O Show InChI InChI=1S/C19H22N2O5.2ClH/c20-6-3-7-21-10-12(22)11-25-13-8-15(23)18-17(9-13)26-16-5-2-1-4-14(16)19(18)24;;/h1-2,4-5,8-9,12,21-23H,3,6-7,10-11,20H2;2*1H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmacy and Biotechnology, Alma Mater Studiorum-University of Bologna, Via Belmeloro 6, 40126 Bologna, Italy; Laboratory of Molecular Modeling and Drug Discovery, Istituto Italiano di
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human topoisomerase-2alpha expressed in Saccharomyces cerevisiae JEL1 harboring topoisomerase1 deletion mutant assessed as ... |
Bioorg Med Chem Lett 27: 4687-4693 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.011
BindingDB Entry DOI: 10.7270/Q21R6T0Q |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597276

(CHEMBL5177294)Show SMILES [H][C@@]1(OC[C@@H](CO1)NC(=O)c1ccc2OCC(=O)Nc2n1)[C@@H](O)Cc1c(F)cnc2ccc(OC)nc12 |r,wD:21.24,4.7,1.0,(-1.88,-1.31,;-1.49,.18,;-.73,1.51,;.81,1.5,;1.57,.17,;.8,-1.15,;-.72,-1.14,;3.11,.17,;3.88,1.5,;3.11,2.83,;5.42,1.5,;6.18,.16,;7.71,.15,;8.49,1.48,;10.02,1.47,;10.8,2.8,;10.04,4.14,;10.81,5.48,;8.49,4.15,;7.71,2.82,;6.18,2.82,;-3.01,.18,;-3.34,1.68,;-4.16,-.85,;-4.15,-2.39,;-2.82,-3.15,;-1.5,-2.37,;-2.81,-4.7,;-4.14,-5.47,;-5.47,-4.7,;-6.81,-5.48,;-8.14,-4.71,;-8.14,-3.16,;-9.48,-2.4,;-10.81,-3.17,;-6.81,-2.4,;-5.47,-3.16,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597275
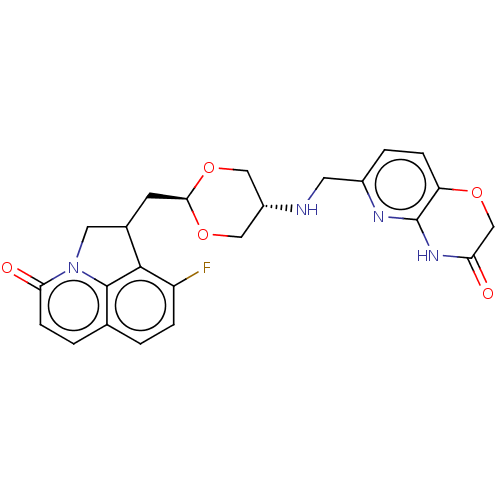
(CHEMBL5197372)Show SMILES Fc1ccc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4ccc5OCC(=O)Nc5n4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-2.51,;-3.04,-3.28,;-3.04,-4.82,;-4.37,-5.59,;-5.7,-4.82,;-7.03,-5.59,;-8.37,-4.82,;-8.37,-3.28,;-9.7,-2.51,;-7.03,-2.51,;-6.47,-1.08,;-4.93,-1.08,;-4.16,.25,;-2.62,.25,;-1.85,1.59,;-.31,1.59,;.46,.25,;-.31,-1.08,;-1.85,-1.08,;2,.25,;2.77,1.59,;4.31,1.59,;5.08,.25,;6.62,.25,;7.39,1.59,;8.92,1.59,;9.7,2.92,;8.93,4.26,;9.7,5.59,;7.38,4.26,;6.62,2.93,;5.08,2.93,;-4.37,-2.51,;-5.7,-3.28,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597277
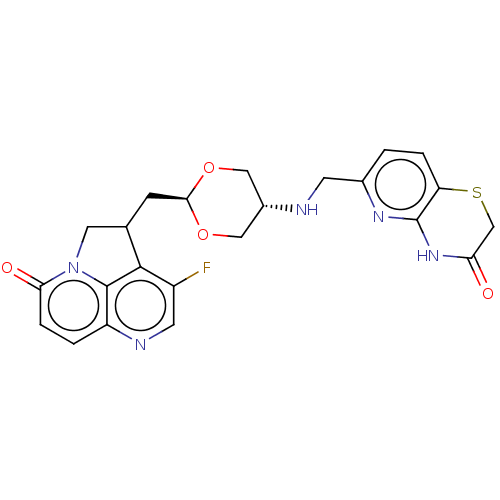
(CHEMBL5208691)Show SMILES Fc1cnc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4ccc5SCC(=O)Nc5n4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-2.51,;-3.04,-3.28,;-3.04,-4.82,;-4.37,-5.59,;-5.7,-4.82,;-7.03,-5.59,;-8.37,-4.82,;-8.37,-3.28,;-9.7,-2.51,;-7.03,-2.51,;-6.47,-1.08,;-4.93,-1.08,;-4.16,.25,;-2.62,.25,;-1.85,1.59,;-.31,1.59,;.46,.25,;-.31,-1.08,;-1.85,-1.08,;2,.25,;2.77,1.59,;4.31,1.59,;5.08,.25,;6.62,.25,;7.39,1.59,;8.92,1.59,;9.7,2.92,;8.93,4.26,;9.7,5.59,;7.38,4.26,;6.62,2.93,;5.08,2.93,;-4.37,-2.51,;-5.7,-3.28,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597274
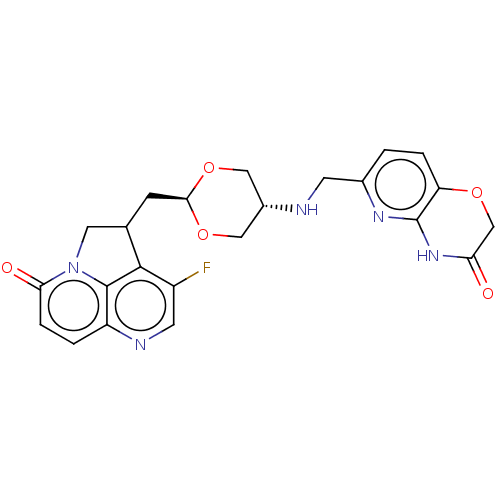
(CHEMBL5183589)Show SMILES Fc1cnc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4ccc5OCC(=O)Nc5n4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-2.51,;-3.04,-3.28,;-3.04,-4.82,;-4.37,-5.59,;-5.7,-4.82,;-7.03,-5.59,;-8.37,-4.82,;-8.37,-3.28,;-9.7,-2.51,;-7.03,-2.51,;-6.47,-1.08,;-4.93,-1.08,;-4.16,.25,;-2.62,.25,;-1.85,1.59,;-.31,1.59,;.46,.25,;-.31,-1.08,;-1.85,-1.08,;2,.25,;2.77,1.59,;4.31,1.59,;5.08,.25,;6.62,.25,;7.39,1.59,;8.92,1.59,;9.7,2.92,;8.93,4.26,;9.7,5.59,;7.38,4.26,;6.62,2.93,;5.08,2.93,;-4.37,-2.51,;-5.7,-3.28,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597275

(CHEMBL5197372)Show SMILES Fc1ccc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4ccc5OCC(=O)Nc5n4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-2.51,;-3.04,-3.28,;-3.04,-4.82,;-4.37,-5.59,;-5.7,-4.82,;-7.03,-5.59,;-8.37,-4.82,;-8.37,-3.28,;-9.7,-2.51,;-7.03,-2.51,;-6.47,-1.08,;-4.93,-1.08,;-4.16,.25,;-2.62,.25,;-1.85,1.59,;-.31,1.59,;.46,.25,;-.31,-1.08,;-1.85,-1.08,;2,.25,;2.77,1.59,;4.31,1.59,;5.08,.25,;6.62,.25,;7.39,1.59,;8.92,1.59,;9.7,2.92,;8.93,4.26,;9.7,5.59,;7.38,4.26,;6.62,2.93,;5.08,2.93,;-4.37,-2.51,;-5.7,-3.28,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597269
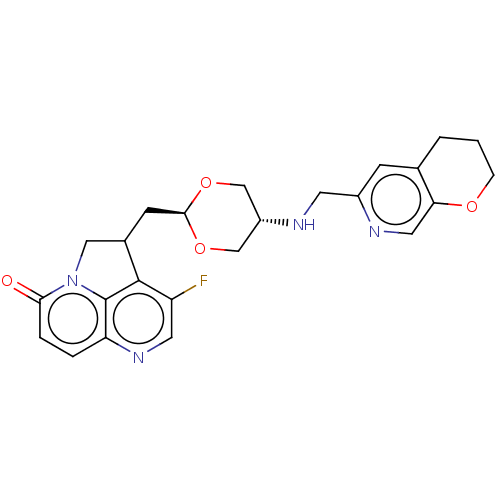
(CHEMBL5190565)Show SMILES Fc1cnc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4cc5CCCOc5cn4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.85,;-3.03,-2.62,;-3.03,-4.16,;-4.37,-4.93,;-5.7,-4.16,;-7.03,-4.93,;-8.37,-4.16,;-8.37,-2.62,;-9.7,-1.85,;-7.03,-1.85,;-6.47,-.41,;-4.93,-.41,;-4.16,.91,;-2.62,.91,;-1.85,2.25,;-.31,2.25,;.46,.91,;-.31,-.41,;-1.85,-.41,;2,.91,;2.77,2.25,;4.31,2.25,;5.08,3.59,;6.62,3.59,;7.38,4.93,;8.93,4.92,;9.7,3.58,;8.92,2.25,;7.39,2.25,;6.62,.91,;5.08,.91,;-4.37,-1.85,;-5.7,-2.62,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM159897
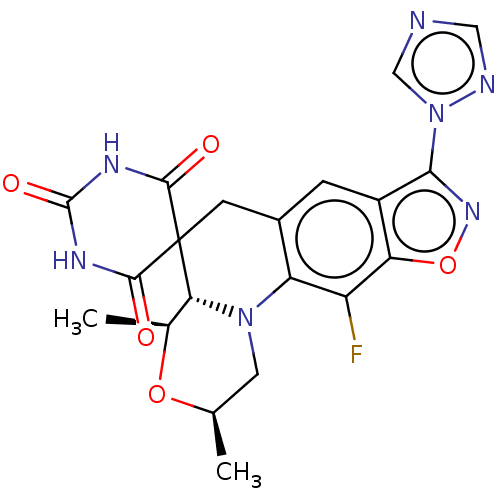
(BDBM159898 | US9040528, 53)Show SMILES C[C@@H]1CN2[C@H]([C@H](C)O1)C1(Cc3cc4c(noc4c(F)c23)-n2cncn2)C(=O)NC(=O)NC1=O |r| Show InChI InChI=1S/C20H18FN7O5/c1-8-5-27-13-10(3-11-14(12(13)21)33-26-16(11)28-7-22-6-23-28)4-20(15(27)9(2)32-8)17(29)24-19(31)25-18(20)30/h3,6-9,15H,4-5H2,1-2H3,(H2,24,25,29,30,31)/t8-,9+,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00266
BindingDB Entry DOI: 10.7270/Q2C251HR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597268

(CHEMBL5171625)Show SMILES [H][C@@]1(OC[C@@H](CO1)NC(=O)c1ccc2OCCOc2c1)[C@@H](O)Cc1c(F)cnc2ccc(OC)nc12 |r,wD:20.23,4.7,1.0,(-1.88,-.64,;-1.49,.84,;-.73,2.18,;.81,2.16,;1.57,.84,;.8,-.49,;-.72,-.48,;3.11,.83,;3.89,2.16,;3.12,3.5,;5.43,2.16,;6.19,.82,;7.72,.81,;8.5,2.15,;10.03,2.14,;10.81,3.47,;10.05,4.81,;8.5,4.82,;7.72,3.49,;6.19,3.49,;-3.01,.84,;-3.34,2.35,;-4.16,-.19,;-4.15,-1.73,;-2.82,-2.49,;-1.49,-1.71,;-2.81,-4.04,;-4.14,-4.81,;-5.48,-4.04,;-6.81,-4.82,;-8.15,-4.05,;-8.14,-2.5,;-9.48,-1.74,;-10.81,-2.51,;-6.81,-1.74,;-5.48,-2.5,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597272
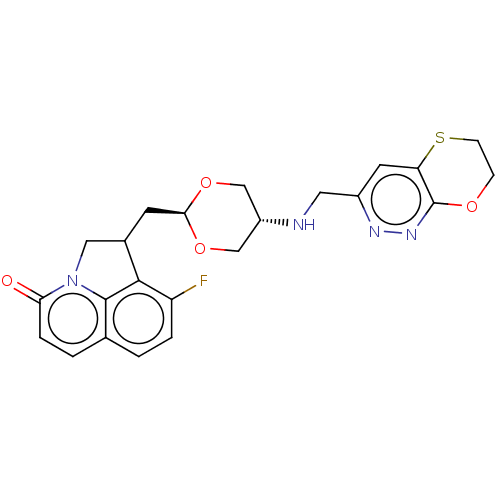
(CHEMBL5209188)Show SMILES Fc1ccc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4cc5SCCOc5nn4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.85,;-3.03,-2.62,;-3.03,-4.16,;-4.37,-4.93,;-5.7,-4.16,;-7.03,-4.93,;-8.37,-4.16,;-8.37,-2.62,;-9.7,-1.85,;-7.03,-1.85,;-6.47,-.41,;-4.93,-.41,;-4.16,.91,;-2.62,.91,;-1.85,2.25,;-.31,2.25,;.46,.91,;-.31,-.41,;-1.85,-.41,;2,.91,;2.77,2.25,;4.31,2.25,;5.08,3.59,;6.62,3.59,;7.38,4.93,;8.93,4.92,;9.7,3.58,;8.92,2.25,;7.39,2.25,;6.62,.91,;5.08,.91,;-4.37,-1.85,;-5.7,-2.62,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597271
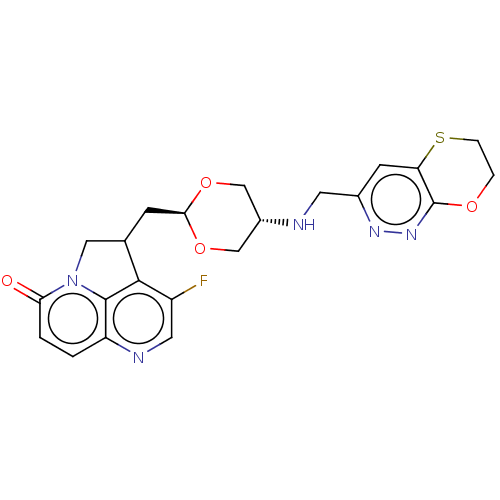
(CHEMBL5204820)Show SMILES Fc1cnc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4cc5SCCOc5nn4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.85,;-3.03,-2.62,;-3.03,-4.16,;-4.37,-4.93,;-5.7,-4.16,;-7.03,-4.93,;-8.37,-4.16,;-8.37,-2.62,;-9.7,-1.85,;-7.03,-1.85,;-6.47,-.41,;-4.93,-.41,;-4.16,.91,;-2.62,.91,;-1.85,2.25,;-.31,2.25,;.46,.91,;-.31,-.41,;-1.85,-.41,;2,.91,;2.77,2.25,;4.31,2.25,;5.08,3.59,;6.62,3.59,;7.38,4.93,;8.93,4.92,;9.7,3.58,;8.92,2.25,;7.39,2.25,;6.62,.91,;5.08,.91,;-4.37,-1.85,;-5.7,-2.62,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50554356
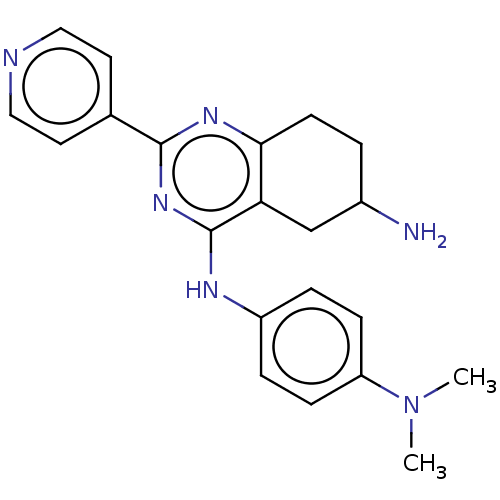
(CHEMBL4758733)Show SMILES CN(C)c1ccc(Nc2nc(nc3CCC(N)Cc23)-c2ccncc2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant wild-type human topoisomerase 2alpha expressed in Saccharomyces cerevisiae using supercoiled pBR322 DNA as substrate measur... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00774
BindingDB Entry DOI: 10.7270/Q26H4N2X |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50554356

(CHEMBL4758733)Show SMILES CN(C)c1ccc(Nc2nc(nc3CCC(N)Cc23)-c2ccncc2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human topoisomerase 2alpha using supercoiled pBR322 DNA as substrate incubated for 60 mins by ethidium bromide dye based ag... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00774
BindingDB Entry DOI: 10.7270/Q26H4N2X |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50605628
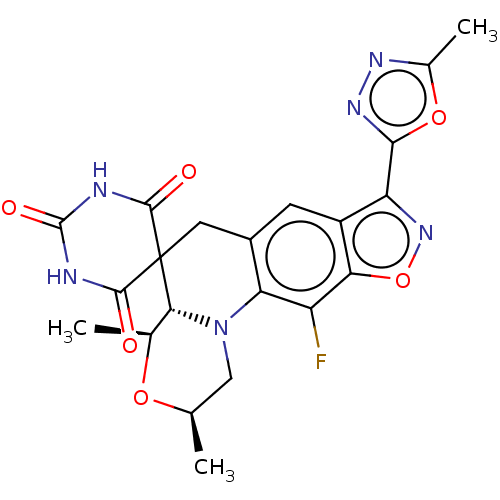
(CHEMBL5189511)Show SMILES [H][C@]12[C@H](C)O[C@H](C)CN1c1c(CC22C(=O)NC(=O)NC2=O)cc2c(noc2c1F)-c1nnc(C)o1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00266
BindingDB Entry DOI: 10.7270/Q2C251HR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597277

(CHEMBL5208691)Show SMILES Fc1cnc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4ccc5SCC(=O)Nc5n4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-2.51,;-3.04,-3.28,;-3.04,-4.82,;-4.37,-5.59,;-5.7,-4.82,;-7.03,-5.59,;-8.37,-4.82,;-8.37,-3.28,;-9.7,-2.51,;-7.03,-2.51,;-6.47,-1.08,;-4.93,-1.08,;-4.16,.25,;-2.62,.25,;-1.85,1.59,;-.31,1.59,;.46,.25,;-.31,-1.08,;-1.85,-1.08,;2,.25,;2.77,1.59,;4.31,1.59,;5.08,.25,;6.62,.25,;7.39,1.59,;8.92,1.59,;9.7,2.92,;8.93,4.26,;9.7,5.59,;7.38,4.26,;6.62,2.93,;5.08,2.93,;-4.37,-2.51,;-5.7,-3.28,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597269

(CHEMBL5190565)Show SMILES Fc1cnc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4cc5CCCOc5cn4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.85,;-3.03,-2.62,;-3.03,-4.16,;-4.37,-4.93,;-5.7,-4.16,;-7.03,-4.93,;-8.37,-4.16,;-8.37,-2.62,;-9.7,-1.85,;-7.03,-1.85,;-6.47,-.41,;-4.93,-.41,;-4.16,.91,;-2.62,.91,;-1.85,2.25,;-.31,2.25,;.46,.91,;-.31,-.41,;-1.85,-.41,;2,.91,;2.77,2.25,;4.31,2.25,;5.08,3.59,;6.62,3.59,;7.38,4.93,;8.93,4.92,;9.7,3.58,;8.92,2.25,;7.39,2.25,;6.62,.91,;5.08,.91,;-4.37,-1.85,;-5.7,-2.62,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50261761
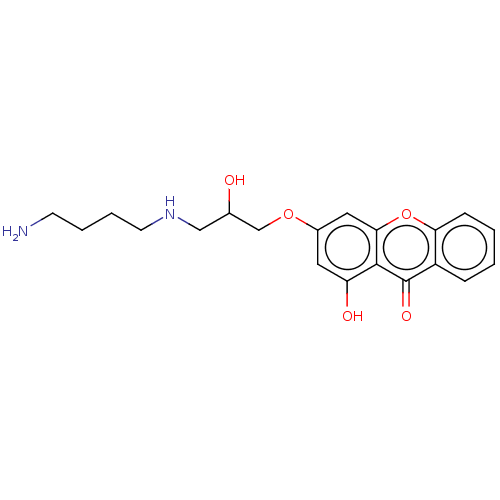
(CHEMBL4096982)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.NCCCCNCC(O)COc1cc(O)c2c(c1)oc1ccccc1c2=O Show InChI InChI=1S/C20H24N2O5.2C2HF3O2/c21-7-3-4-8-22-11-13(23)12-26-14-9-16(24)19-18(10-14)27-17-6-2-1-5-15(17)20(19)25;2*3-2(4,5)1(6)7/h1-2,5-6,9-10,13,22-24H,3-4,7-8,11-12,21H2;2*(H,6,7) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmacy and Biotechnology, Alma Mater Studiorum-University of Bologna, Via Belmeloro 6, 40126 Bologna, Italy; Laboratory of Molecular Modeling and Drug Discovery, Istituto Italiano di
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human topoisomerase-2alpha expressed in Saccharomyces cerevisiae JEL1 harboring topoisomerase1 deletion mutant assessed as ... |
Bioorg Med Chem Lett 27: 4687-4693 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.011
BindingDB Entry DOI: 10.7270/Q21R6T0Q |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50605623
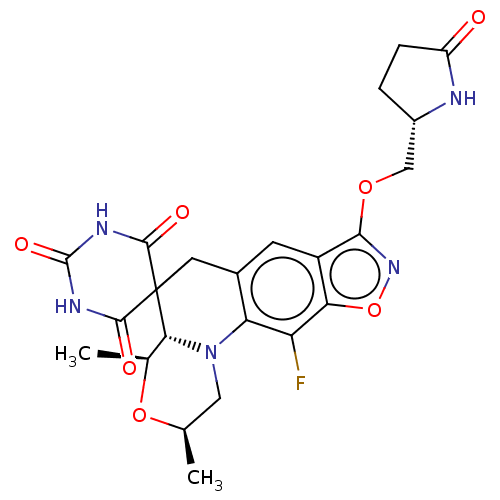
(CHEMBL5203837)Show SMILES [H][C@]12[C@H](C)O[C@H](C)CN1c1c(CC22C(=O)NC(=O)NC2=O)cc2c(OC[C@@H]3CCC(=O)N3)noc2c1F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00266
BindingDB Entry DOI: 10.7270/Q2C251HR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597279

(CHEMBL4537408)Show SMILES O=c1ccc2ncc(=O)n3C(CN4CCC(CC4)NCc4cc5CCCOc5cn4)Cn1c23 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597270

(CHEMBL5195074)Show SMILES Fc1ccc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4cc5CCCOc5cn4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.85,;-3.03,-2.62,;-3.03,-4.16,;-4.37,-4.93,;-5.7,-4.16,;-7.03,-4.93,;-8.37,-4.16,;-8.37,-2.62,;-9.7,-1.85,;-7.03,-1.85,;-6.47,-.41,;-4.93,-.41,;-4.16,.91,;-2.62,.91,;-1.85,2.25,;-.31,2.25,;.46,.91,;-.31,-.41,;-1.85,-.41,;2,.91,;2.77,2.25,;4.31,2.25,;5.08,3.59,;6.62,3.59,;7.38,4.93,;8.93,4.92,;9.7,3.58,;8.92,2.25,;7.39,2.25,;6.62,.91,;5.08,.91,;-4.37,-1.85,;-5.7,-2.62,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50605622
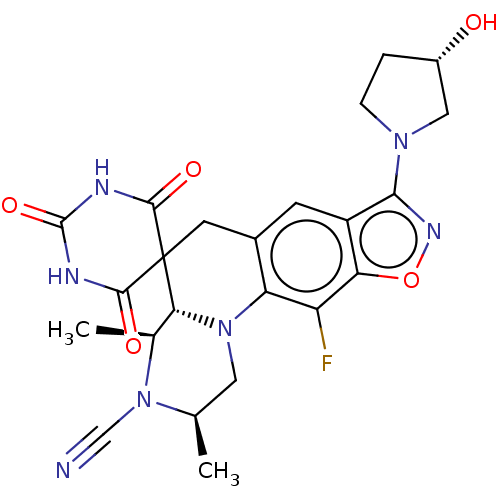
(CHEMBL5183469)Show SMILES [H][C@]12[C@H](C)N(C#N)[C@H](C)CN1c1c(CC22C(=O)NC(=O)NC2=O)cc2c(noc2c1F)N1CC[C@H](O)C1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00266
BindingDB Entry DOI: 10.7270/Q2C251HR |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597273
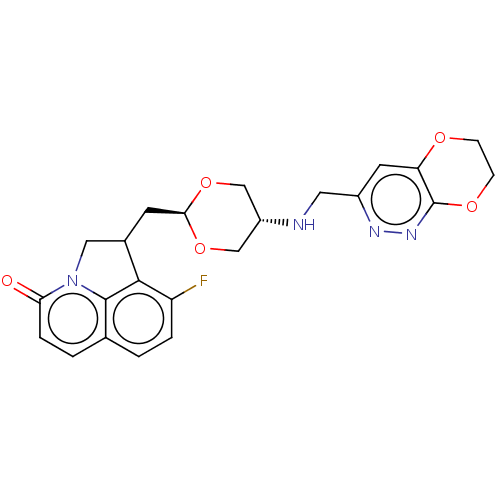
(CHEMBL5197477)Show SMILES Fc1ccc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4cc5OCCOc5nn4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.85,;-3.03,-2.62,;-3.03,-4.16,;-4.37,-4.93,;-5.7,-4.16,;-7.03,-4.93,;-8.37,-4.16,;-8.37,-2.62,;-9.7,-1.85,;-7.03,-1.85,;-6.47,-.41,;-4.93,-.41,;-4.16,.91,;-2.62,.91,;-1.85,2.25,;-.31,2.25,;.46,.91,;-.31,-.41,;-1.85,-.41,;2,.91,;2.77,2.25,;4.31,2.25,;5.08,3.59,;6.62,3.59,;7.38,4.93,;8.93,4.92,;9.7,3.58,;8.92,2.25,;7.39,2.25,;6.62,.91,;5.08,.91,;-4.37,-1.85,;-5.7,-2.62,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50554356

(CHEMBL4758733)Show SMILES CN(C)c1ccc(Nc2nc(nc3CCC(N)Cc23)-c2ccncc2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human COX1 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00774
BindingDB Entry DOI: 10.7270/Q26H4N2X |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50597274

(CHEMBL5183589)Show SMILES Fc1cnc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4ccc5OCC(=O)Nc5n4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-2.51,;-3.04,-3.28,;-3.04,-4.82,;-4.37,-5.59,;-5.7,-4.82,;-7.03,-5.59,;-8.37,-4.82,;-8.37,-3.28,;-9.7,-2.51,;-7.03,-2.51,;-6.47,-1.08,;-4.93,-1.08,;-4.16,.25,;-2.62,.25,;-1.85,1.59,;-.31,1.59,;.46,.25,;-.31,-1.08,;-1.85,-1.08,;2,.25,;2.77,1.59,;4.31,1.59,;5.08,.25,;6.62,.25,;7.39,1.59,;8.92,1.59,;9.7,2.92,;8.93,4.26,;9.7,5.59,;7.38,4.26,;6.62,2.93,;5.08,2.93,;-4.37,-2.51,;-5.7,-3.28,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM139376
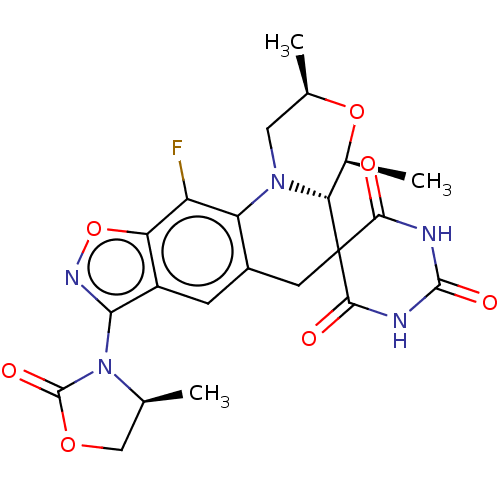
(US8889671, 5)Show SMILES C[C@H]1COC(=O)N1c1noc2c(F)c3N4C[C@@H](C)O[C@@H](C)[C@@H]4C4(Cc3cc12)C(=O)NC(=O)NC4=O |r| Show InChI InChI=1S/C22H22FN5O7/c1-8-7-33-21(32)28(8)17-12-4-11-5-22(18(29)24-20(31)25-19(22)30)16-10(3)34-9(2)6-27(16)14(11)13(23)15(12)35-26-17/h4,8-10,16H,5-7H2,1-3H3,(H2,24,25,29,30,31)/t8-,9+,10-,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00266
BindingDB Entry DOI: 10.7270/Q2C251HR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50152149
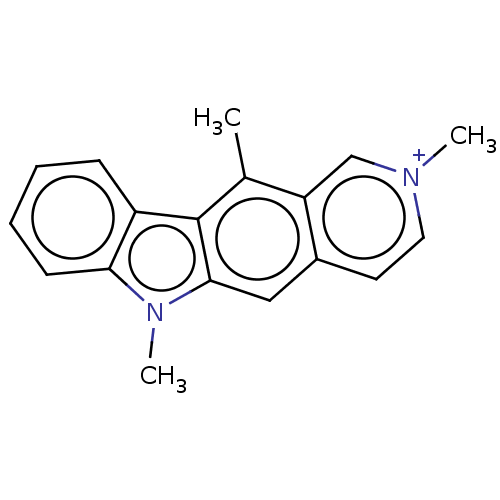
(CHEMBL3780961)Show InChI InChI=1S/C18H17N2.HI/c1-12-15-11-19(2)9-8-13(15)10-17-18(12)14-6-4-5-7-16(14)20(17)3;/h4-11H,1-3H3;1H/q+1;/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DNA topoisomerase 2alpha expressed in topoisomerase1 deficient Saccharomyces cerevisiae JEL1 assessed as inhibition o... |
Bioorg Med Chem Lett 26: 1809-12 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.034
BindingDB Entry DOI: 10.7270/Q2MC91WG |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50605621
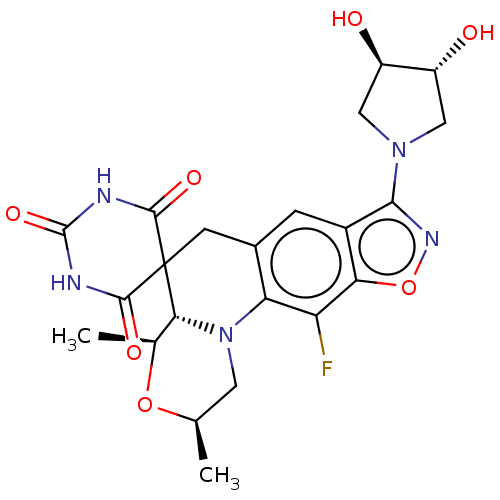
(CHEMBL5206134)Show SMILES [H][C@]12[C@H](C)O[C@H](C)CN1c1c(CC22C(=O)NC(=O)NC2=O)cc2c(noc2c1F)N1C[C@@H](O)[C@H](O)C1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00266
BindingDB Entry DOI: 10.7270/Q2C251HR |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50366824
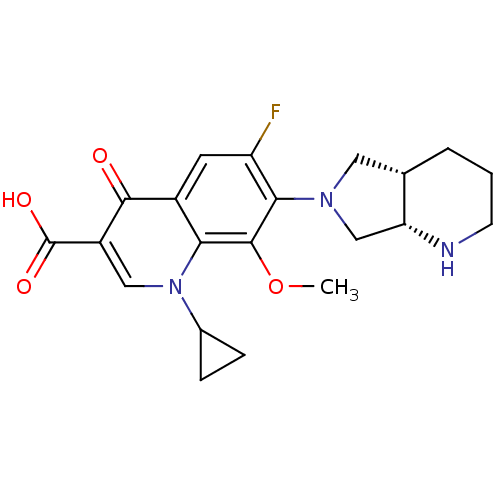
(Avelox | MOXIFLOXACIN | Moxifl-oxacin)Show SMILES COc1c(N2C[C@@H]3CCCN[C@@H]3C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 |r| Show InChI InChI=1S/C21H24FN3O4/c1-29-20-17-13(19(26)14(21(27)28)9-25(17)12-4-5-12)7-15(22)18(20)24-8-11-3-2-6-23-16(11)10-24/h7,9,11-12,16,23H,2-6,8,10H2,1H3,(H,27,28)/t11-,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00266
BindingDB Entry DOI: 10.7270/Q2C251HR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50605620
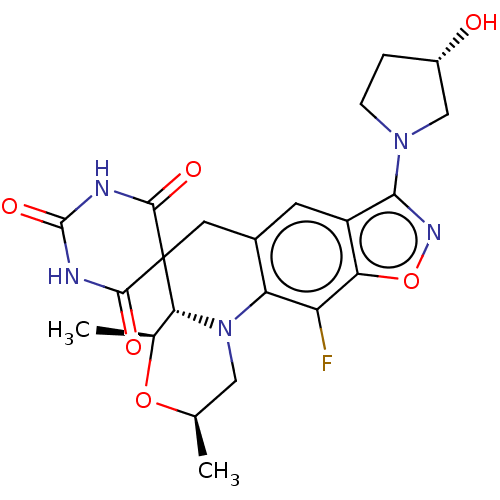
(CHEMBL5201644)Show SMILES [H][C@]12[C@H](C)O[C@H](C)CN1c1c(CC22C(=O)NC(=O)NC2=O)cc2c(noc2c1F)N1CC[C@H](O)C1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00266
BindingDB Entry DOI: 10.7270/Q2C251HR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597272

(CHEMBL5209188)Show SMILES Fc1ccc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4cc5SCCOc5nn4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.85,;-3.03,-2.62,;-3.03,-4.16,;-4.37,-4.93,;-5.7,-4.16,;-7.03,-4.93,;-8.37,-4.16,;-8.37,-2.62,;-9.7,-1.85,;-7.03,-1.85,;-6.47,-.41,;-4.93,-.41,;-4.16,.91,;-2.62,.91,;-1.85,2.25,;-.31,2.25,;.46,.91,;-.31,-.41,;-1.85,-.41,;2,.91,;2.77,2.25,;4.31,2.25,;5.08,3.59,;6.62,3.59,;7.38,4.93,;8.93,4.92,;9.7,3.58,;8.92,2.25,;7.39,2.25,;6.62,.91,;5.08,.91,;-4.37,-1.85,;-5.7,-2.62,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597273

(CHEMBL5197477)Show SMILES Fc1ccc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4cc5OCCOc5nn4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.85,;-3.03,-2.62,;-3.03,-4.16,;-4.37,-4.93,;-5.7,-4.16,;-7.03,-4.93,;-8.37,-4.16,;-8.37,-2.62,;-9.7,-1.85,;-7.03,-1.85,;-6.47,-.41,;-4.93,-.41,;-4.16,.91,;-2.62,.91,;-1.85,2.25,;-.31,2.25,;.46,.91,;-.31,-.41,;-1.85,-.41,;2,.91,;2.77,2.25,;4.31,2.25,;5.08,3.59,;6.62,3.59,;7.38,4.93,;8.93,4.92,;9.7,3.58,;8.92,2.25,;7.39,2.25,;6.62,.91,;5.08,.91,;-4.37,-1.85,;-5.7,-2.62,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50261769

(CHEMBL4070811)Show SMILES Cl.Cl.Cl.Cl.NCCCNCCCCNCCCNCC(O)COc1cc(O)c2c(c1)oc1ccccc1c2=O Show InChI InChI=1S/C26H38N4O5.4ClH/c27-9-5-12-28-10-3-4-11-29-13-6-14-30-17-19(31)18-34-20-15-22(32)25-24(16-20)35-23-8-2-1-7-21(23)26(25)33;;;;/h1-2,7-8,15-16,19,28-32H,3-6,9-14,17-18,27H2;4*1H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmacy and Biotechnology, Alma Mater Studiorum-University of Bologna, Via Belmeloro 6, 40126 Bologna, Italy; Laboratory of Molecular Modeling and Drug Discovery, Istituto Italiano di
Curated by ChEMBL
| Assay Description
Inhibition of 150 nM recombinant human topoisomerase-2alpha catalytic activity expressed in Saccharomyces cerevisiae JEL1 harboring topoisomerase1 de... |
Bioorg Med Chem Lett 27: 4687-4693 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.011
BindingDB Entry DOI: 10.7270/Q21R6T0Q |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50261770
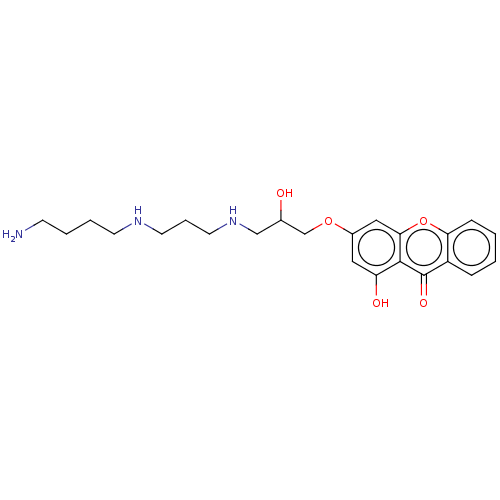
(CHEMBL4069784)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.NCCCCNCCCNCC(O)COc1cc(O)c2c(c1)oc1ccccc1c2=O Show InChI InChI=1S/C23H31N3O5.3C2HF3O2/c24-8-3-4-9-25-10-5-11-26-14-16(27)15-30-17-12-19(28)22-21(13-17)31-20-7-2-1-6-18(20)23(22)29;3*3-2(4,5)1(6)7/h1-2,6-7,12-13,16,25-28H,3-5,8-11,14-15,24H2;3*(H,6,7) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmacy and Biotechnology, Alma Mater Studiorum-University of Bologna, Via Belmeloro 6, 40126 Bologna, Italy; Laboratory of Molecular Modeling and Drug Discovery, Istituto Italiano di
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human topoisomerase-2alpha expressed in Saccharomyces cerevisiae JEL1 harboring topoisomerase1 deletion mutant assessed as ... |
Bioorg Med Chem Lett 27: 4687-4693 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.011
BindingDB Entry DOI: 10.7270/Q21R6T0Q |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50261770

(CHEMBL4069784)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.NCCCCNCCCNCC(O)COc1cc(O)c2c(c1)oc1ccccc1c2=O Show InChI InChI=1S/C23H31N3O5.3C2HF3O2/c24-8-3-4-9-25-10-5-11-26-14-16(27)15-30-17-12-19(28)22-21(13-17)31-20-7-2-1-6-18(20)23(22)29;3*3-2(4,5)1(6)7/h1-2,6-7,12-13,16,25-28H,3-5,8-11,14-15,24H2;3*(H,6,7) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmacy and Biotechnology, Alma Mater Studiorum-University of Bologna, Via Belmeloro 6, 40126 Bologna, Italy; Laboratory of Molecular Modeling and Drug Discovery, Istituto Italiano di
Curated by ChEMBL
| Assay Description
Inhibition of 150 nM recombinant human topoisomerase-2alpha catalytic activity expressed in Saccharomyces cerevisiae JEL1 harboring topoisomerase1 de... |
Bioorg Med Chem Lett 27: 4687-4693 (2017)
Article DOI: 10.1016/j.bmcl.2017.09.011
BindingDB Entry DOI: 10.7270/Q21R6T0Q |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50597271

(CHEMBL5204820)Show SMILES Fc1cnc2ccc(=O)n3CC(C[C@H]4OC[C@@H](CO4)NCc4cc5SCCOc5nn4)c1c23 |r,wU:13.12,wD:16.19,(-1.7,-1.85,;-3.03,-2.62,;-3.03,-4.16,;-4.37,-4.93,;-5.7,-4.16,;-7.03,-4.93,;-8.37,-4.16,;-8.37,-2.62,;-9.7,-1.85,;-7.03,-1.85,;-6.47,-.41,;-4.93,-.41,;-4.16,.91,;-2.62,.91,;-1.85,2.25,;-.31,2.25,;.46,.91,;-.31,-.41,;-1.85,-.41,;2,.91,;2.77,2.25,;4.31,2.25,;5.08,3.59,;6.62,3.59,;7.38,4.93,;8.93,4.92,;9.7,3.58,;8.92,2.25,;7.39,2.25,;6.62,.91,;5.08,.91,;-4.37,-1.85,;-5.7,-2.62,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00111
BindingDB Entry DOI: 10.7270/Q2TT4W0M |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50605627
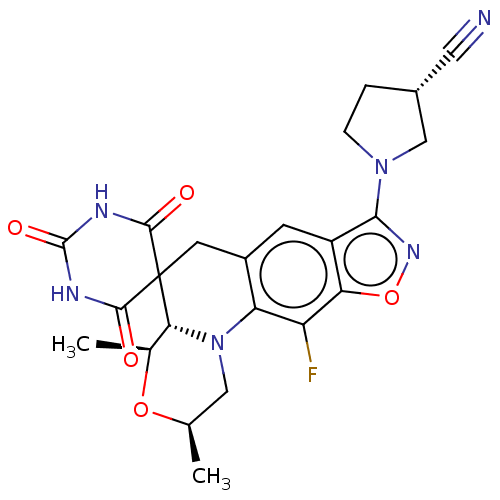
(CHEMBL5186061)Show SMILES [H][C@]12[C@H](C)O[C@H](C)CN1c1c(CC22C(=O)NC(=O)NC2=O)cc2c(noc2c1F)N1CC[C@@H](C1)C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00266
BindingDB Entry DOI: 10.7270/Q2C251HR |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50366824

(Avelox | MOXIFLOXACIN | Moxifl-oxacin)Show SMILES COc1c(N2C[C@@H]3CCCN[C@@H]3C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 |r| Show InChI InChI=1S/C21H24FN3O4/c1-29-20-17-13(19(26)14(21(27)28)9-25(17)12-4-5-12)7-15(22)18(20)24-8-11-3-2-6-23-16(11)10-24/h7,9,11-12,16,23H,2-6,8,10H2,1H3,(H,27,28)/t11-,16+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00266
BindingDB Entry DOI: 10.7270/Q2C251HR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50605621

(CHEMBL5206134)Show SMILES [H][C@]12[C@H](C)O[C@H](C)CN1c1c(CC22C(=O)NC(=O)NC2=O)cc2c(noc2c1F)N1C[C@@H](O)[C@H](O)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00266
BindingDB Entry DOI: 10.7270/Q2C251HR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data