 Found 60 hits with Last Name = 'otsuji' and Initial = 'y'
Found 60 hits with Last Name = 'otsuji' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50384947
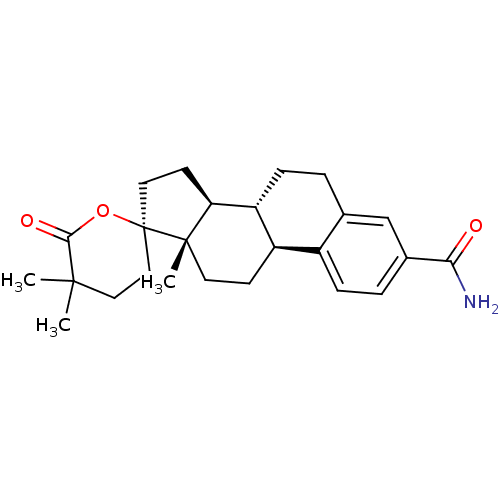
(CHEMBL521703)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(ccc34)C(N)=O)[C@@H]1CC[C@@]21CCC(C)(C)C(=O)O1 |r| Show InChI InChI=1S/C25H33NO3/c1-23(2)12-13-25(29-22(23)28)11-9-20-19-7-4-15-14-16(21(26)27)5-6-17(15)18(19)8-10-24(20,25)3/h5-6,14,18-20H,4,7-13H2,1-3H3,(H2,26,27)/t18-,19-,20+,24+,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human GST-tagged 17betaHSD5 expressed in Escherichia coli by radiometric assay |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM35905
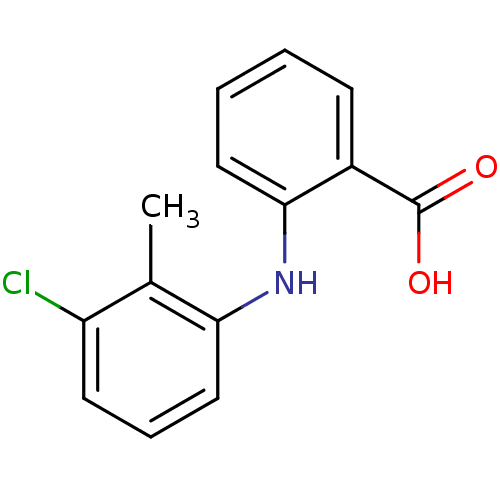
(Tolfenamic acid | cid_610479 | flufenamic acid ana...)Show InChI InChI=1S/C14H12ClNO2/c1-9-11(15)6-4-8-12(9)16-13-7-3-2-5-10(13)14(17)18/h2-8,16H,1H3,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C3 using S-(+)-1,2,3,4-tetrahydro-1-naphthol as substrate |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50384946
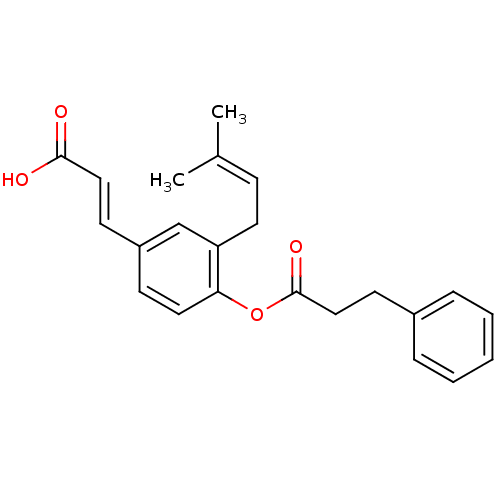
(CHEMBL511708)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](-[#8])=O)ccc1-[#8]-[#6](=O)-[#6]-[#6]-c1ccccc1 Show InChI InChI=1S/C23H24O4/c1-17(2)8-12-20-16-19(10-14-22(24)25)9-13-21(20)27-23(26)15-11-18-6-4-3-5-7-18/h3-10,13-14,16H,11-12,15H2,1-2H3,(H,24,25)/b14-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50384946

(CHEMBL511708)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](-[#8])=O)ccc1-[#8]-[#6](=O)-[#6]-[#6]-c1ccccc1 Show InChI InChI=1S/C23H24O4/c1-17(2)8-12-20-16-19(10-14-22(24)25)9-13-21(20)27-23(26)15-11-18-6-4-3-5-7-18/h3-10,13-14,16H,11-12,15H2,1-2H3,(H,24,25)/b14-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using NADP+ linked S-tetralol as substrate by fluorometr... |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50384946

(CHEMBL511708)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](-[#8])=O)ccc1-[#8]-[#6](=O)-[#6]-[#6]-c1ccccc1 Show InChI InChI=1S/C23H24O4/c1-17(2)8-12-20-16-19(10-14-22(24)25)9-13-21(20)27-23(26)15-11-18-6-4-3-5-7-18/h3-10,13-14,16H,11-12,15H2,1-2H3,(H,24,25)/b14-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry in prese... |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50362836
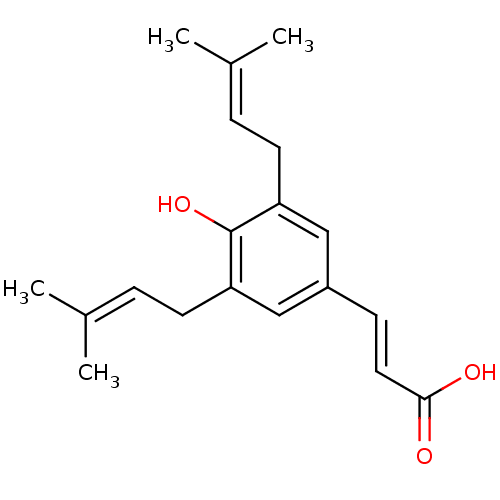
(ARTEPILLIN)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](-[#8])=O)cc(-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8] Show InChI InChI=1S/C19H24O3/c1-13(2)5-8-16-11-15(7-10-18(20)21)12-17(19(16)22)9-6-14(3)4/h5-7,10-12,22H,8-9H2,1-4H3,(H,20,21)/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry in prese... |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50362836

(ARTEPILLIN)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](-[#8])=O)cc(-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8] Show InChI InChI=1S/C19H24O3/c1-13(2)5-8-16-11-15(7-10-18(20)21)12-17(19(16)22)9-6-14(3)4/h5-7,10-12,22H,8-9H2,1-4H3,(H,20,21)/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024764
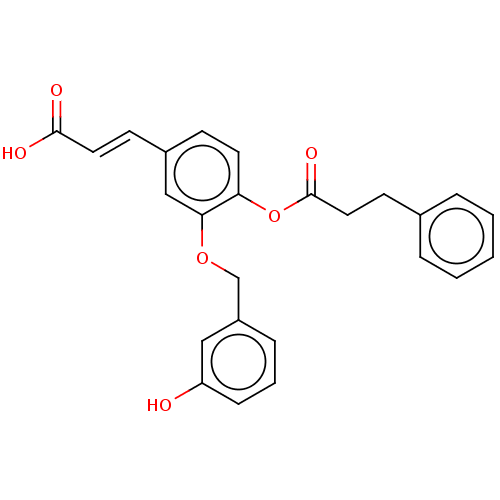
(CHEMBL3337721)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2ccccc2)c(OCc2cccc(O)c2)c1 Show InChI InChI=1S/C25H22O6/c26-21-8-4-7-20(15-21)17-30-23-16-19(10-13-24(27)28)9-12-22(23)31-25(29)14-11-18-5-2-1-3-6-18/h1-10,12-13,15-16,26H,11,14,17H2,(H,27,28)/b13-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50384946

(CHEMBL511708)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](-[#8])=O)ccc1-[#8]-[#6](=O)-[#6]-[#6]-c1ccccc1 Show InChI InChI=1S/C23H24O4/c1-17(2)8-12-20-16-19(10-14-22(24)25)9-13-21(20)27-23(26)15-11-18-6-4-3-5-7-18/h3-10,13-14,16H,11-12,15H2,1-2H3,(H,24,25)/b14-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50384946

(CHEMBL511708)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](-[#8])=O)ccc1-[#8]-[#6](=O)-[#6]-[#6]-c1ccccc1 Show InChI InChI=1S/C23H24O4/c1-17(2)8-12-20-16-19(10-14-22(24)25)9-13-21(20)27-23(26)15-11-18-6-4-3-5-7-18/h3-10,13-14,16H,11-12,15H2,1-2H3,(H,24,25)/b14-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024782

(CHEMBL3337705)Show SMILES Cc1ccc(COc2cc(\C=C\C(O)=O)ccc2OC(=O)CCc2ccccc2)cc1 Show InChI InChI=1S/C26H24O5/c1-19-7-9-22(10-8-19)18-30-24-17-21(12-15-25(27)28)11-14-23(24)31-26(29)16-13-20-5-3-2-4-6-20/h2-12,14-15,17H,13,16,18H2,1H3,(H,27,28)/b15-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024780
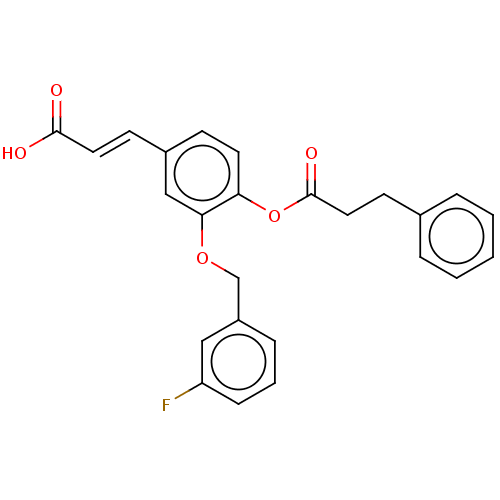
(CHEMBL3337707)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2ccccc2)c(OCc2cccc(F)c2)c1 Show InChI InChI=1S/C25H21FO5/c26-21-8-4-7-20(15-21)17-30-23-16-19(10-13-24(27)28)9-12-22(23)31-25(29)14-11-18-5-2-1-3-6-18/h1-10,12-13,15-16H,11,14,17H2,(H,27,28)/b13-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024774
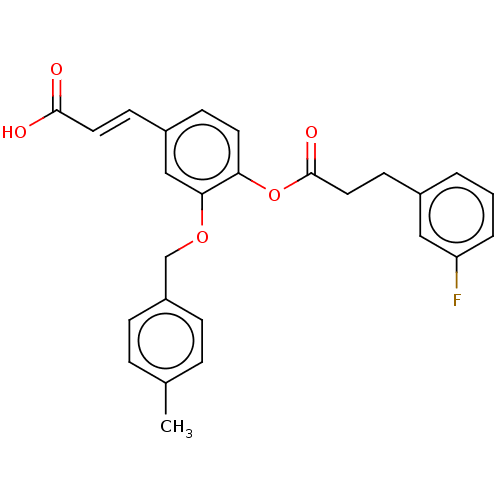
(CHEMBL3337711)Show SMILES Cc1ccc(COc2cc(\C=C\C(O)=O)ccc2OC(=O)CCc2cccc(F)c2)cc1 Show InChI InChI=1S/C26H23FO5/c1-18-5-7-21(8-6-18)17-31-24-16-20(10-13-25(28)29)9-12-23(24)32-26(30)14-11-19-3-2-4-22(27)15-19/h2-10,12-13,15-16H,11,14,17H2,1H3,(H,28,29)/b13-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024778
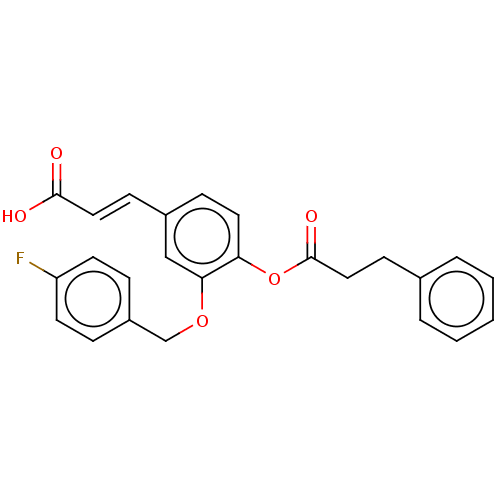
(CHEMBL3337458)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2ccccc2)c(OCc2ccc(F)cc2)c1 Show InChI InChI=1S/C25H21FO5/c26-21-11-6-20(7-12-21)17-30-23-16-19(9-14-24(27)28)8-13-22(23)31-25(29)15-10-18-4-2-1-3-5-18/h1-9,11-14,16H,10,15,17H2,(H,27,28)/b14-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024781

(CHEMBL3337706)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2ccccc2)c(OCc2ccccc2F)c1 Show InChI InChI=1S/C25H21FO5/c26-21-9-5-4-8-20(21)17-30-23-16-19(11-14-24(27)28)10-13-22(23)31-25(29)15-12-18-6-2-1-3-7-18/h1-11,13-14,16H,12,15,17H2,(H,27,28)/b14-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024777

(CHEMBL3337708)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2ccccc2)c(OCc2cc(F)cc(F)c2)c1 Show InChI InChI=1S/C25H20F2O5/c26-20-12-19(13-21(27)15-20)16-31-23-14-18(7-10-24(28)29)6-9-22(23)32-25(30)11-8-17-4-2-1-3-5-17/h1-7,9-10,12-15H,8,11,16H2,(H,28,29)/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024783

(CHEMBL3337704)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2ccccc2)c(OCc2ccccc2)c1 Show InChI InChI=1S/C25H22O5/c26-24(27)15-12-20-11-14-22(23(17-20)29-18-21-9-5-2-6-10-21)30-25(28)16-13-19-7-3-1-4-8-19/h1-12,14-15,17H,13,16,18H2,(H,26,27)/b15-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024769

(CHEMBL3337716)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2cccc(F)c2)c(OCc2cccc(F)c2)c1 Show InChI InChI=1S/C25H20F2O5/c26-20-5-1-3-17(13-20)9-12-25(30)32-22-10-7-18(8-11-24(28)29)15-23(22)31-16-19-4-2-6-21(27)14-19/h1-8,10-11,13-15H,9,12,16H2,(H,28,29)/b11-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024787
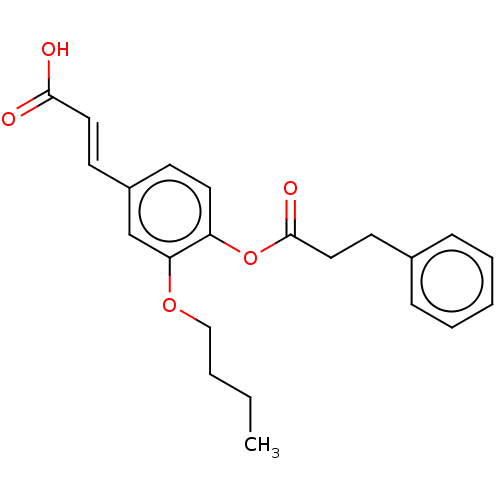
(CHEMBL3337700)Show InChI InChI=1S/C22H24O5/c1-2-3-15-26-20-16-18(10-13-21(23)24)9-12-19(20)27-22(25)14-11-17-7-5-4-6-8-17/h4-10,12-13,16H,2-3,11,14-15H2,1H3,(H,23,24)/b13-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024773
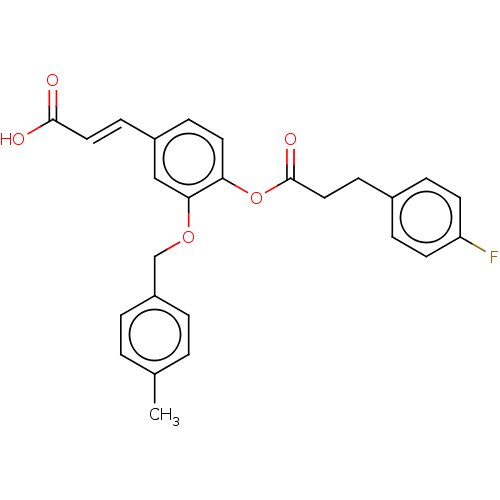
(CHEMBL3337712)Show SMILES Cc1ccc(COc2cc(\C=C\C(O)=O)ccc2OC(=O)CCc2ccc(F)cc2)cc1 Show InChI InChI=1S/C26H23FO5/c1-18-2-4-21(5-3-18)17-31-24-16-20(9-14-25(28)29)8-13-23(24)32-26(30)15-10-19-6-11-22(27)12-7-19/h2-9,11-14,16H,10,15,17H2,1H3,(H,28,29)/b14-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024772

(CHEMBL3337713)Show SMILES Cc1ccc(COc2cc(\C=C\C(O)=O)ccc2OC(=O)CCc2c(F)cc(F)cc2F)cc1 Show InChI InChI=1S/C26H21F3O5/c1-16-2-4-18(5-3-16)15-33-24-12-17(7-10-25(30)31)6-9-23(24)34-26(32)11-8-20-21(28)13-19(27)14-22(20)29/h2-7,9-10,12-14H,8,11,15H2,1H3,(H,30,31)/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024768

(CHEMBL3337717)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2ccc(F)cc2)c(OCc2cccc(F)c2)c1 Show InChI InChI=1S/C25H20F2O5/c26-20-9-4-17(5-10-20)8-13-25(30)32-22-11-6-18(7-12-24(28)29)15-23(22)31-16-19-2-1-3-21(27)14-19/h1-7,9-12,14-15H,8,13,16H2,(H,28,29)/b12-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024786

(CHEMBL3337701)Show SMILES CC(C)COc1cc(\C=C\C(O)=O)ccc1OC(=O)CCc1ccccc1 Show InChI InChI=1S/C22H24O5/c1-16(2)15-26-20-14-18(9-12-21(23)24)8-11-19(20)27-22(25)13-10-17-6-4-3-5-7-17/h3-9,11-12,14,16H,10,13,15H2,1-2H3,(H,23,24)/b12-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024775

(CHEMBL3337710)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2ccccc2)c(OCCc2ccccc2)c1 Show InChI InChI=1S/C26H24O5/c27-25(28)15-12-22-11-14-23(31-26(29)16-13-20-7-3-1-4-8-20)24(19-22)30-18-17-21-9-5-2-6-10-21/h1-12,14-15,19H,13,16-18H2,(H,27,28)/b15-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024767

(CHEMBL3337718)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2c(F)cc(F)cc2F)c(OCc2cccc(F)c2)c1 Show InChI InChI=1S/C25H18F4O5/c26-17-3-1-2-16(10-17)14-33-23-11-15(5-8-24(30)31)4-7-22(23)34-25(32)9-6-19-20(28)12-18(27)13-21(19)29/h1-5,7-8,10-13H,6,9,14H2,(H,30,31)/b8-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024771
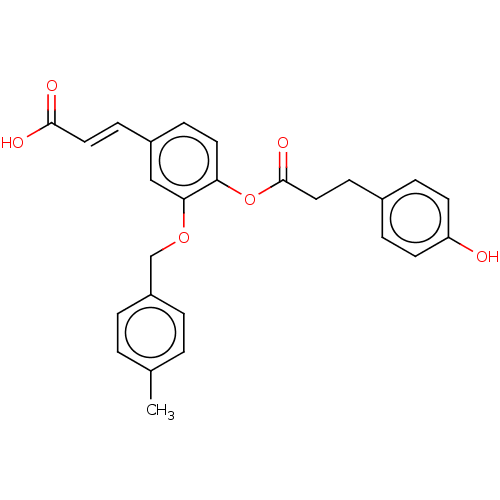
(CHEMBL3337714)Show SMILES Cc1ccc(COc2cc(\C=C\C(O)=O)ccc2OC(=O)CCc2ccc(O)cc2)cc1 Show InChI InChI=1S/C26H24O6/c1-18-2-4-21(5-3-18)17-31-24-16-20(9-14-25(28)29)8-13-23(24)32-26(30)15-10-19-6-11-22(27)12-7-19/h2-9,11-14,16,27H,10,15,17H2,1H3,(H,28,29)/b14-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024766

(CHEMBL3337719)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2cccc(O)c2)c(OCc2cccc(F)c2)c1 Show InChI InChI=1S/C25H21FO6/c26-20-5-1-4-19(13-20)16-31-23-15-18(8-11-24(28)29)7-10-22(23)32-25(30)12-9-17-3-2-6-21(27)14-17/h1-8,10-11,13-15,27H,9,12,16H2,(H,28,29)/b11-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024765

(CHEMBL3337720)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2ccc(O)cc2)c(OCc2cccc(F)c2)c1 Show InChI InChI=1S/C25H21FO6/c26-20-3-1-2-19(14-20)16-31-23-15-18(7-12-24(28)29)6-11-22(23)32-25(30)13-8-17-4-9-21(27)10-5-17/h1-7,9-12,14-15,27H,8,13,16H2,(H,28,29)/b12-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024784

(CHEMBL3337703)Show SMILES CC(=C)COc1cc(\C=C\C(O)=O)ccc1OC(=O)CCc1ccccc1 Show InChI InChI=1S/C22H22O5/c1-16(2)15-26-20-14-18(9-12-21(23)24)8-11-19(20)27-22(25)13-10-17-6-4-3-5-7-17/h3-9,11-12,14H,1,10,13,15H2,2H3,(H,23,24)/b12-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024776

(CHEMBL3337709)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2ccccc2)c(OCc2ccc(Cl)cc2)c1 Show InChI InChI=1S/C25H21ClO5/c26-21-11-6-20(7-12-21)17-30-23-16-19(9-14-24(27)28)8-13-22(23)31-25(29)15-10-18-4-2-1-3-5-18/h1-9,11-14,16H,10,15,17H2,(H,27,28)/b14-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024770

(CHEMBL3337715)Show SMILES Cc1ccc(COc2cc(\C=C\C(O)=O)ccc2OC(=O)CCc2cccc(O)c2)cc1 Show InChI InChI=1S/C26H24O6/c1-18-5-7-21(8-6-18)17-31-24-16-20(10-13-25(28)29)9-12-23(24)32-26(30)14-11-19-3-2-4-22(27)15-19/h2-10,12-13,15-16,27H,11,14,17H2,1H3,(H,28,29)/b13-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024763
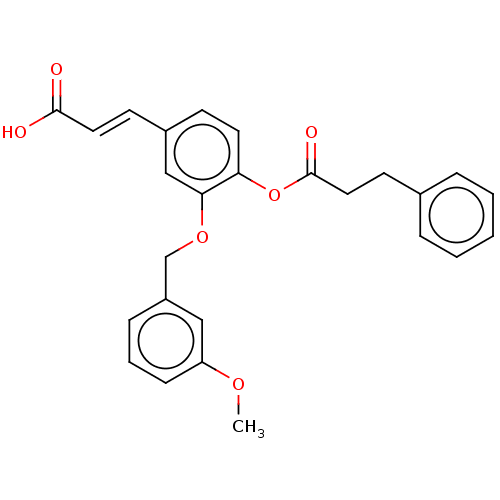
(CHEMBL3337722)Show SMILES COc1cccc(COc2cc(\C=C\C(O)=O)ccc2OC(=O)CCc2ccccc2)c1 Show InChI InChI=1S/C26H24O6/c1-30-22-9-5-8-21(16-22)18-31-24-17-20(11-14-25(27)28)10-13-23(24)32-26(29)15-12-19-6-3-2-4-7-19/h2-11,13-14,16-17H,12,15,18H2,1H3,(H,27,28)/b14-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024793
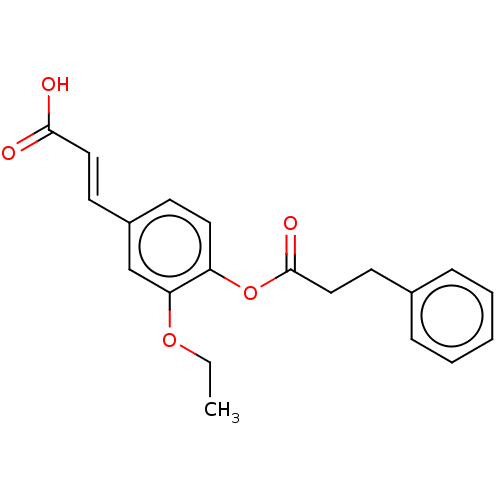
(CHEMBL3337697)Show InChI InChI=1S/C20H20O5/c1-2-24-18-14-16(9-12-19(21)22)8-11-17(18)25-20(23)13-10-15-6-4-3-5-7-15/h3-9,11-12,14H,2,10,13H2,1H3,(H,21,22)/b12-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50362836

(ARTEPILLIN)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](-[#8])=O)cc(-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8] Show InChI InChI=1S/C19H24O3/c1-13(2)5-8-16-11-15(7-10-18(20)21)12-17(19(16)22)9-6-14(3)4/h5-7,10-12,22H,8-9H2,1-4H3,(H,20,21)/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024785

(CHEMBL3337702)Show SMILES C\C=C\COc1cc(\C=C\C(O)=O)ccc1OC(=O)CCc1ccccc1 Show InChI InChI=1S/C22H22O5/c1-2-3-15-26-20-16-18(10-13-21(23)24)9-12-19(20)27-22(25)14-11-17-7-5-4-6-8-17/h2-10,12-13,16H,11,14-15H2,1H3,(H,23,24)/b3-2+,13-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50029207

((E)-3-(3,4-Dihydroxy-phenyl)-acrylic acid phenethy...)Show InChI InChI=1S/C17H16O4/c18-15-8-6-14(12-16(15)19)7-9-17(20)21-11-10-13-4-2-1-3-5-13/h1-9,12,18-19H,10-11H2/b9-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024789
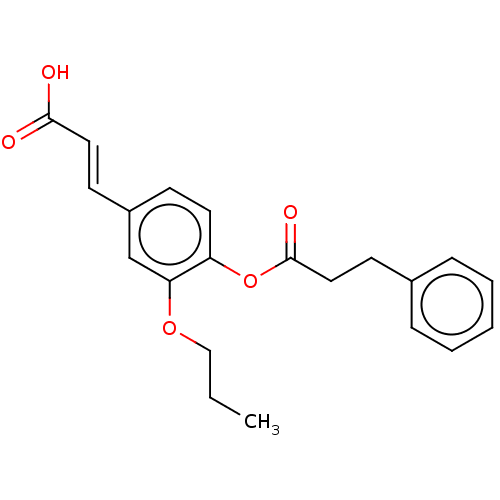
(CHEMBL3337698)Show InChI InChI=1S/C21H22O5/c1-2-14-25-19-15-17(9-12-20(22)23)8-11-18(19)26-21(24)13-10-16-6-4-3-5-7-16/h3-9,11-12,15H,2,10,13-14H2,1H3,(H,22,23)/b12-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C4
(Homo sapiens (Human)) | BDBM50029207

((E)-3-(3,4-Dihydroxy-phenyl)-acrylic acid phenethy...)Show InChI InChI=1S/C17H16O4/c18-15-8-6-14(12-16(15)19)7-9-17(20)21-11-10-13-4-2-1-3-5-13/h1-9,12,18-19H,10-11H2/b9-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged AKR1C4 expressed in Escherichia coli using S-tetralol as substrate by fluorometry |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024788
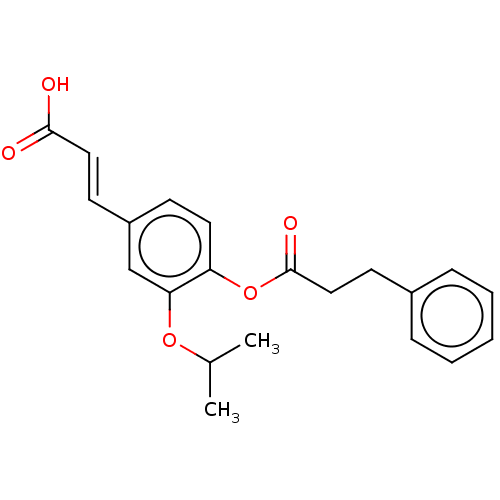
(CHEMBL3337699)Show SMILES CC(C)Oc1cc(\C=C\C(O)=O)ccc1OC(=O)CCc1ccccc1 Show InChI InChI=1S/C21H22O5/c1-15(2)25-19-14-17(9-12-20(22)23)8-11-18(19)26-21(24)13-10-16-6-4-3-5-7-16/h3-9,11-12,14-15H,10,13H2,1-2H3,(H,22,23)/b12-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024796

(CHEMBL3337696)Show InChI InChI=1S/C19H18O5/c1-23-17-13-15(8-11-18(20)21)7-10-16(17)24-19(22)12-9-14-5-3-2-4-6-14/h2-8,10-11,13H,9,12H2,1H3,(H,20,21)/b11-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50029207

((E)-3-(3,4-Dihydroxy-phenyl)-acrylic acid phenethy...)Show InChI InChI=1S/C17H16O4/c18-15-8-6-14(12-16(15)19)7-9-17(20)21-11-10-13-4-2-1-3-5-13/h1-9,12,18-19H,10-11H2/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C2 expressed in Escherichia coli using S-tetralol as substrate by fluorometry |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50024764

(CHEMBL3337721)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2ccccc2)c(OCc2cccc(O)c2)c1 Show InChI InChI=1S/C25H22O6/c26-21-8-4-7-20(15-21)17-30-23-16-19(10-13-24(27)28)9-12-22(23)31-25(29)14-11-18-5-2-1-3-6-18/h1-10,12-13,15-16,26H,11,14,17H2,(H,27,28)/b13-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3-mediated androsterone metabolism in human A549 cells assessed as 5alpha-androstane-3alpha, 17beta-diol after 24 hrs by LC/MS ana... |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50029207

((E)-3-(3,4-Dihydroxy-phenyl)-acrylic acid phenethy...)Show InChI InChI=1S/C17H16O4/c18-15-8-6-14(12-16(15)19)7-9-17(20)21-11-10-13-4-2-1-3-5-13/h1-9,12,18-19H,10-11H2/b9-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged AKR1C1 expressed in Escherichia coli using S-tetralol as substrate by fluorometry |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50362837
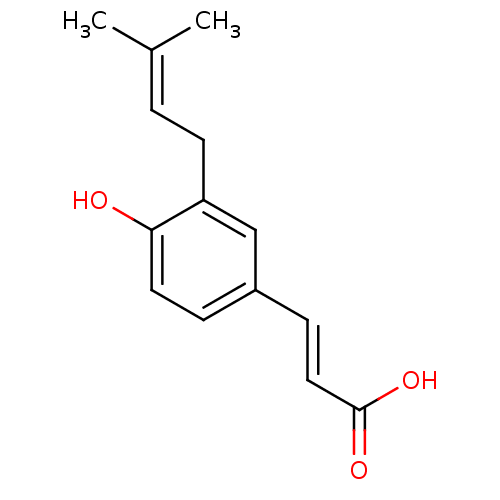
(DRUPANIN)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](-[#8])=O)ccc1-[#8] Show InChI InChI=1S/C14H16O3/c1-10(2)3-6-12-9-11(4-7-13(12)15)5-8-14(16)17/h3-5,7-9,15H,6H2,1-2H3,(H,16,17)/b8-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometry |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50384946

(CHEMBL511708)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](-[#8])=O)ccc1-[#8]-[#6](=O)-[#6]-[#6]-c1ccccc1 Show InChI InChI=1S/C23H24O4/c1-17(2)8-12-20-16-19(10-14-22(24)25)9-13-21(20)27-23(26)15-11-18-6-4-3-5-7-18/h3-10,13-14,16H,11-12,15H2,1-2H3,(H,24,25)/b14-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3-mediated androsterone metabolism in human A549 cells assessed as 5alpha-androstane-3alpha, 17beta-diol after 24 hrs by LC/MS ana... |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C4
(Homo sapiens (Human)) | BDBM50024764

(CHEMBL3337721)Show SMILES OC(=O)\C=C\c1ccc(OC(=O)CCc2ccccc2)c(OCc2cccc(O)c2)c1 Show InChI InChI=1S/C25H22O6/c26-21-8-4-7-20(15-21)17-30-23-16-19(10-13-24(27)28)9-12-22(23)31-25(29)14-11-18-5-2-1-3-6-18/h1-10,12-13,15-16,26H,11,14,17H2,(H,27,28)/b13-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C4 (unknown origin) assessed as dehydrogenase activity of enzyme by NADPH fluorescence assay |
Bioorg Med Chem 22: 5220-33 (2014)
Article DOI: 10.1016/j.bmc.2014.08.007
BindingDB Entry DOI: 10.7270/Q28P623F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50384946

(CHEMBL511708)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(\[#6]=[#6]\[#6](-[#8])=O)ccc1-[#8]-[#6](=O)-[#6]-[#6]-c1ccccc1 Show InChI InChI=1S/C23H24O4/c1-17(2)8-12-20-16-19(10-14-22(24)25)9-13-21(20)27-23(26)15-11-18-6-4-3-5-7-18/h3-10,13-14,16H,11-12,15H2,1-2H3,(H,24,25)/b14-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3-mediated [14C]farnesal metabolism in human MCF7 cells incubated for 2 hrs prior to substrate addition measured after 6 hrs |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C4
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged AKR1C4 expressed in Escherichia coli using S-tetralol as substrate by fluorometry |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C2 expressed in Escherichia coli using S-tetralol as substrate by fluorometry |
J Nat Prod 75: 716-21 (2012)
Article DOI: 10.1021/np201002x
BindingDB Entry DOI: 10.7270/Q2Z320P2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data