 Found 33 hits with Last Name = 'owman' and Initial = 'c'
Found 33 hits with Last Name = 'owman' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50106543
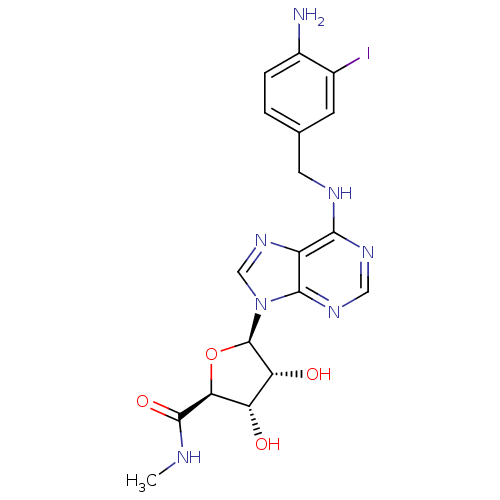
(5-[6-(4-Amino-3-iodo-benzylamino)-purin-9-yl]-3,4-...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3ccc(N)c(I)c3)ncnc12 Show InChI InChI=1S/C18H20IN7O4/c1-21-17(29)14-12(27)13(28)18(30-14)26-7-25-11-15(23-6-24-16(11)26)22-5-8-2-3-10(20)9(19)4-8/h2-4,6-7,12-14,18,27-28H,5,20H2,1H3,(H,21,29)(H,22,23,24)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50207816
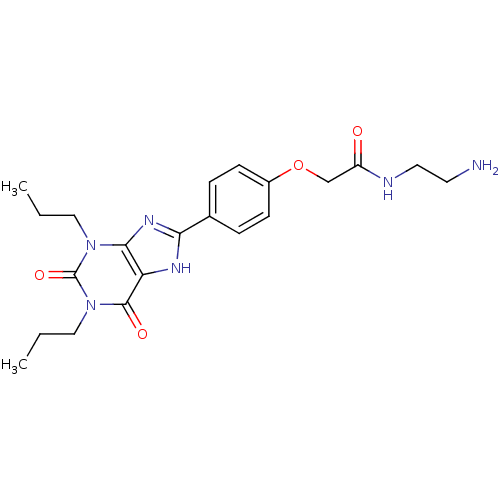
(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50034171
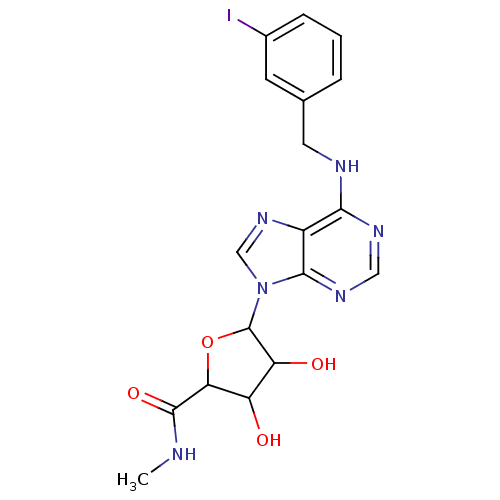
(3,4-Dihydroxy-5-[6-(3-iodo-benzylamino)-purin-9-yl...)Show SMILES CNC(=O)C1OC(C(O)C1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50006730
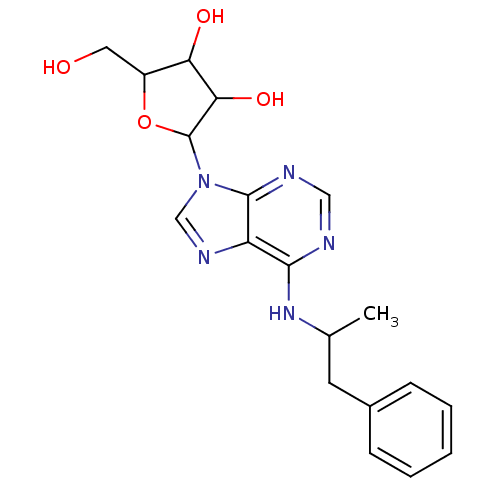
((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21242
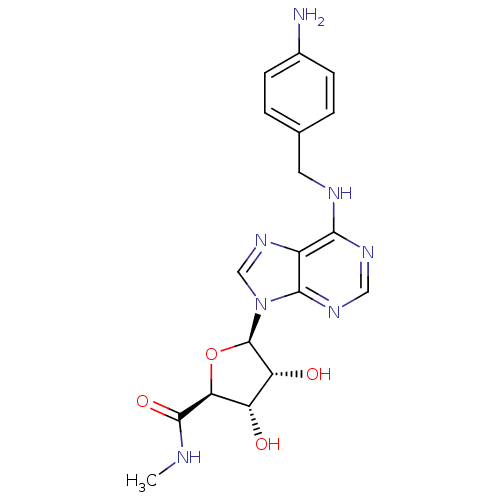
((2S,3S,4R,5R)-5-(6-{[(4-aminophenyl)methyl]amino}-...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3ccc(N)cc3)ncnc12 Show InChI InChI=1S/C18H21N7O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-2-4-10(19)5-3-9/h2-5,7-8,12-14,18,26-27H,6,19H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35804

((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(NCCc3ccc(CCC(O)=O)cc3)nc12 Show InChI InChI=1S/C23H29N7O6/c1-2-25-21(35)18-16(33)17(34)22(36-18)30-11-27-15-19(24)28-23(29-20(15)30)26-10-9-13-5-3-12(4-6-13)7-8-14(31)32/h3-6,11,16-18,22,33-34H,2,7-10H2,1H3,(H,25,35)(H,31,32)(H3,24,26,28,29)/t16-,17+,18-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 27.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50008415
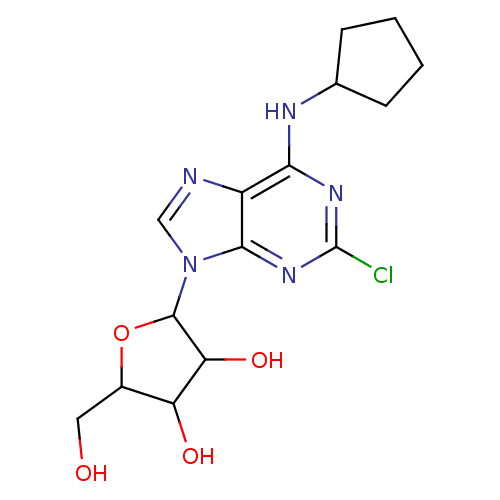
(2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydro...)Show InChI InChI=1S/C15H20ClN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM25400

((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50006730

((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 44.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 50.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM35804

((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(NCCc3ccc(CCC(O)=O)cc3)nc12 Show InChI InChI=1S/C23H29N7O6/c1-2-25-21(35)18-16(33)17(34)22(36-18)30-11-27-15-19(24)28-23(29-20(15)30)26-10-9-13-5-3-12(4-6-13)7-8-14(31)32/h3-6,11,16-18,22,33-34H,2,7-10H2,1H3,(H,25,35)(H,31,32)(H3,24,26,28,29)/t16-,17+,18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 67.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50207816

(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 91.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50106543

(5-[6-(4-Amino-3-iodo-benzylamino)-purin-9-yl]-3,4-...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3ccc(N)c(I)c3)ncnc12 Show InChI InChI=1S/C18H20IN7O4/c1-21-17(29)14-12(27)13(28)18(30-14)26-7-25-11-15(23-6-24-16(11)26)22-5-8-2-3-10(20)9(19)4-8/h2-4,6-7,12-14,18,27-28H,5,20H2,1H3,(H,21,29)(H,22,23,24)/t12-,13+,14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 471 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM25400

((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50006730

((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 859 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM10847
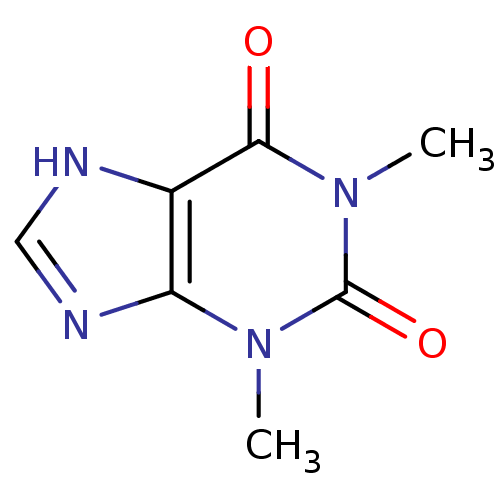
(1,3-dimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-dion...)Show InChI InChI=1S/C7H8N4O2/c1-10-5-4(8-3-9-5)6(12)11(2)7(10)13/h3H,1-2H3,(H,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50008415

(2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydro...)Show InChI InChI=1S/C15H20ClN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50034171

(3,4-Dihydroxy-5-[6-(3-iodo-benzylamino)-purin-9-yl...)Show SMILES CNC(=O)C1OC(C(O)C1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50006730

((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM10847

(1,3-dimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-dion...)Show InChI InChI=1S/C7H8N4O2/c1-10-5-4(8-3-9-5)6(12)11(2)7(10)13/h3H,1-2H3,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 1-9 (1998)
Article DOI: 10.1007/pl00005131
BindingDB Entry DOI: 10.7270/Q20Z71T3 |
More data for this
Ligand-Target Pair | |
Acetylpolyamine amidohydrolase
(Mycoplana ramosa (Gram-negative bacterium)) | BDBM50438390
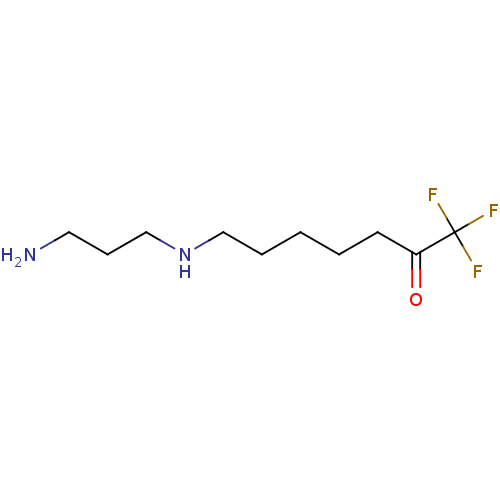
(CHEMBL2414000)Show InChI InChI=1S/C10H19F3N2O/c11-10(12,13)9(16)5-2-1-3-7-15-8-4-6-14/h15H,1-8,14H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assay |
Bioorg Med Chem 21: 4530-40 (2013)
Article DOI: 10.1016/j.bmc.2013.05.045
BindingDB Entry DOI: 10.7270/Q2DN46FQ |
More data for this
Ligand-Target Pair | |
Acetylpolyamine amidohydrolase
(Mycoplana ramosa (Gram-negative bacterium)) | BDBM50438391
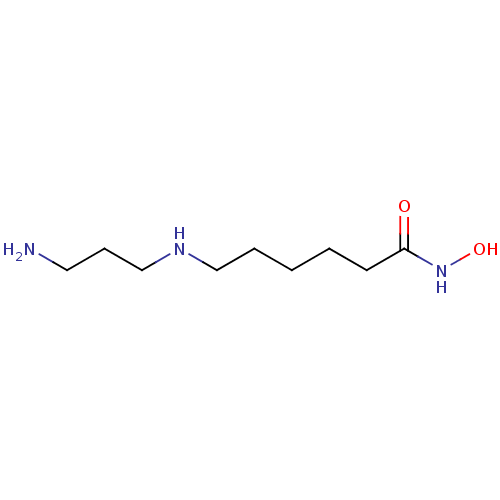
(CHEMBL2413999)Show InChI InChI=1S/C9H21N3O2/c10-6-4-8-11-7-3-1-2-5-9(13)12-14/h11,14H,1-8,10H2,(H,12,13) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assay |
Bioorg Med Chem 21: 4530-40 (2013)
Article DOI: 10.1016/j.bmc.2013.05.045
BindingDB Entry DOI: 10.7270/Q2DN46FQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylpolyamine amidohydrolase
(Mycoplana ramosa (Gram-negative bacterium)) | BDBM50438386
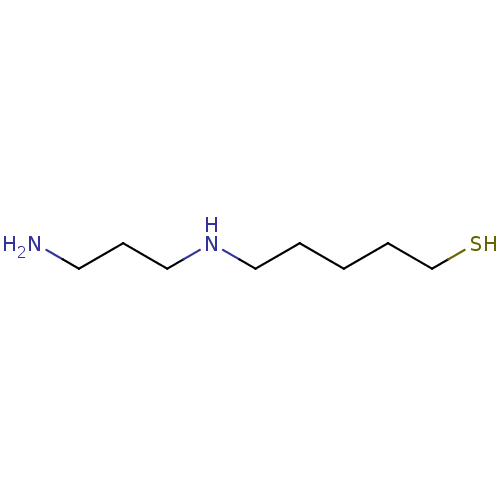
(CHEMBL2414004)Show InChI InChI=1S/C8H20N2S/c9-5-4-7-10-6-2-1-3-8-11/h10-11H,1-9H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assay |
Bioorg Med Chem 21: 4530-40 (2013)
Article DOI: 10.1016/j.bmc.2013.05.045
BindingDB Entry DOI: 10.7270/Q2DN46FQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylpolyamine amidohydrolase
(Mycoplana ramosa (Gram-negative bacterium)) | BDBM50438389
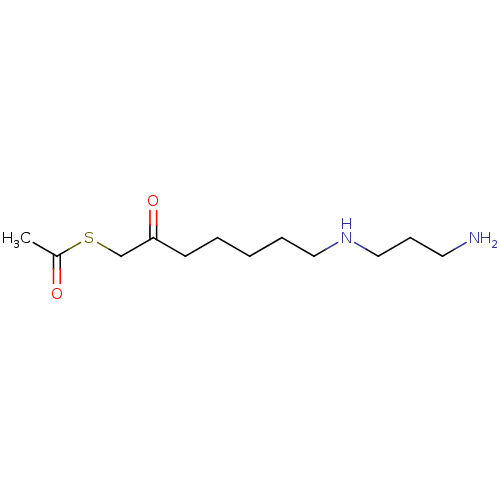
(CHEMBL2414001)Show InChI InChI=1S/C12H24N2O2S/c1-11(15)17-10-12(16)6-3-2-4-8-14-9-5-7-13/h14H,2-10,13H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assay |
Bioorg Med Chem 21: 4530-40 (2013)
Article DOI: 10.1016/j.bmc.2013.05.045
BindingDB Entry DOI: 10.7270/Q2DN46FQ |
More data for this
Ligand-Target Pair | |
Acetylpolyamine amidohydrolase
(Mycoplana ramosa (Gram-negative bacterium)) | BDBM50405937
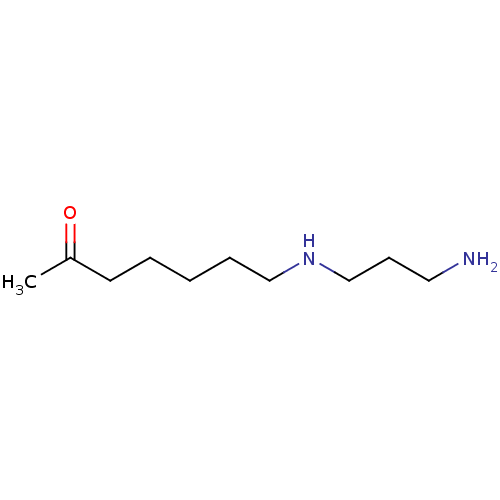
(CHEMBL173782)Show InChI InChI=1S/C10H22N2O/c1-10(13)6-3-2-4-8-12-9-5-7-11/h12H,2-9,11H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assay |
Bioorg Med Chem 21: 4530-40 (2013)
Article DOI: 10.1016/j.bmc.2013.05.045
BindingDB Entry DOI: 10.7270/Q2DN46FQ |
More data for this
Ligand-Target Pair | |
Acetylpolyamine amidohydrolase
(Mycoplana ramosa (Gram-negative bacterium)) | BDBM50438384
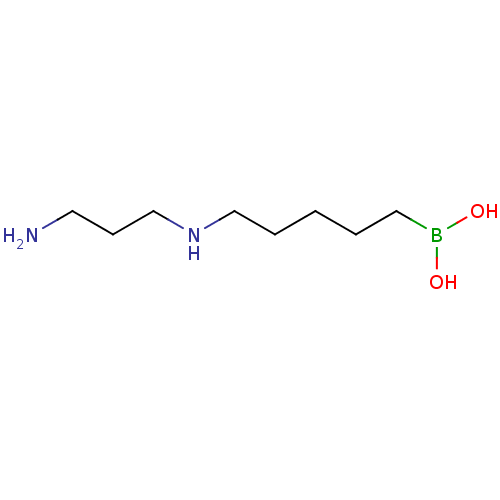
(CHEMBL2414006)Show InChI InChI=1S/C8H21BN2O2/c10-6-4-8-11-7-3-1-2-5-9(12)13/h11-13H,1-8,10H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assay |
Bioorg Med Chem 21: 4530-40 (2013)
Article DOI: 10.1016/j.bmc.2013.05.045
BindingDB Entry DOI: 10.7270/Q2DN46FQ |
More data for this
Ligand-Target Pair | |
Acetylpolyamine amidohydrolase
(Mycoplana ramosa (Gram-negative bacterium)) | BDBM50438388
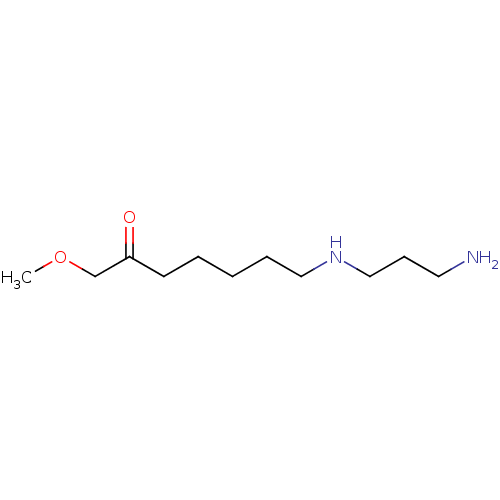
(CHEMBL2414002)Show InChI InChI=1S/C11H24N2O2/c1-15-10-11(14)6-3-2-4-8-13-9-5-7-12/h13H,2-10,12H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assay |
Bioorg Med Chem 21: 4530-40 (2013)
Article DOI: 10.1016/j.bmc.2013.05.045
BindingDB Entry DOI: 10.7270/Q2DN46FQ |
More data for this
Ligand-Target Pair | |
Acetylpolyamine amidohydrolase
(Mycoplana ramosa (Gram-negative bacterium)) | BDBM50438393
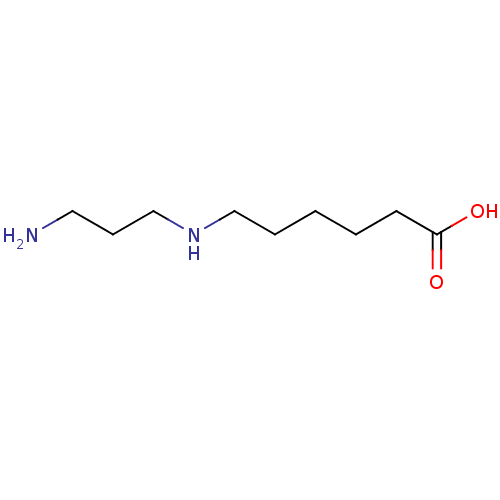
(CHEMBL2414007)Show InChI InChI=1S/C9H20N2O2/c10-6-4-8-11-7-3-1-2-5-9(12)13/h11H,1-8,10H2,(H,12,13) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assay |
Bioorg Med Chem 21: 4530-40 (2013)
Article DOI: 10.1016/j.bmc.2013.05.045
BindingDB Entry DOI: 10.7270/Q2DN46FQ |
More data for this
Ligand-Target Pair | |
Acetylpolyamine amidohydrolase
(Mycoplana ramosa (Gram-negative bacterium)) | BDBM50438387
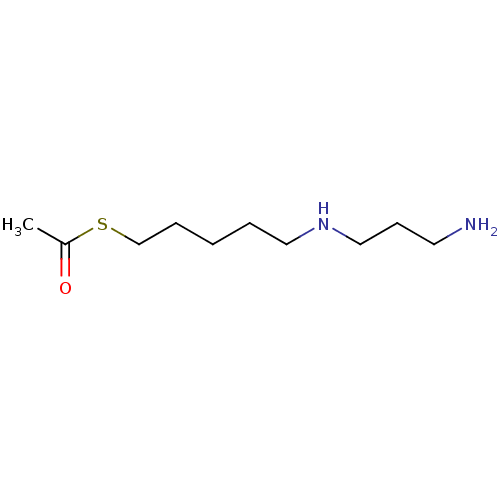
(CHEMBL2414003)Show InChI InChI=1S/C10H22N2OS/c1-10(13)14-9-4-2-3-7-12-8-5-6-11/h12H,2-9,11H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assay |
Bioorg Med Chem 21: 4530-40 (2013)
Article DOI: 10.1016/j.bmc.2013.05.045
BindingDB Entry DOI: 10.7270/Q2DN46FQ |
More data for this
Ligand-Target Pair | |
Acetylpolyamine amidohydrolase
(Mycoplana ramosa (Gram-negative bacterium)) | BDBM50438385
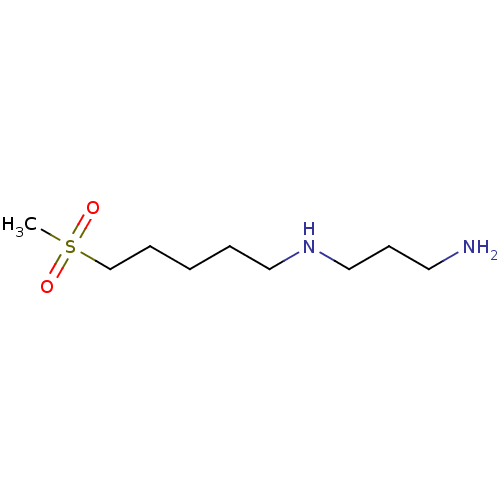
(CHEMBL2414005)Show InChI InChI=1S/C9H22N2O2S/c1-14(12,13)9-4-2-3-7-11-8-5-6-10/h11H,2-10H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Mycoplana ramosa APAH expressed in Escherichia coli BL21 (DE3) using BML-KI104 as substrate after 30 mins by fluorimetric assay |
Bioorg Med Chem 21: 4530-40 (2013)
Article DOI: 10.1016/j.bmc.2013.05.045
BindingDB Entry DOI: 10.7270/Q2DN46FQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data