

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cruzipain (Trypanosoma cruzi) | BDBM50225450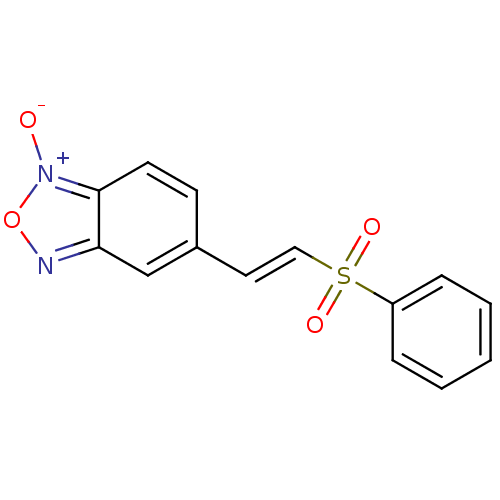 (5(E)-[2-(phenylsulfonyl)vinyl]benzo[1,2-c]1,2,5-ox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de la República Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi Tulahuen 2 cruzipain | J Med Chem 50: 6004-15 (2007) Article DOI: 10.1021/jm070604e BindingDB Entry DOI: 10.7270/Q2NG4QB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50225446 (5(Z)-[2-(phenylsulfonyl)vinyl]benzo[1,2-c]1,2,5-ox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de la República Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi Tulahuen 2 cruzipain | J Med Chem 50: 6004-15 (2007) Article DOI: 10.1021/jm070604e BindingDB Entry DOI: 10.7270/Q2NG4QB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50202581 (CHEMBL218795 | N-hexadecylsulfamide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Activation of human PPARalpha by GST pull down assay | J Med Chem 50: 389-93 (2007) Article DOI: 10.1021/jm0601102 BindingDB Entry DOI: 10.7270/Q2HX1CB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50202582 (CHEMBL374948 | N-oleylsulfamide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 524 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Activation of human PPARalpha by GST pull down assay | J Med Chem 50: 389-93 (2007) Article DOI: 10.1021/jm0601102 BindingDB Entry DOI: 10.7270/Q2HX1CB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM29080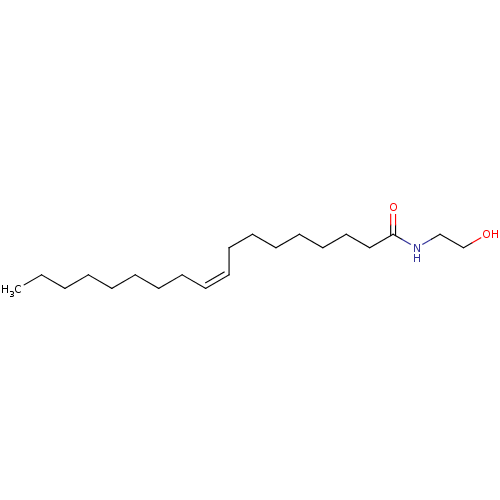 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Activation of human PPARalpha by GST pull down assay | J Med Chem 50: 389-93 (2007) Article DOI: 10.1021/jm0601102 BindingDB Entry DOI: 10.7270/Q2HX1CB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50202583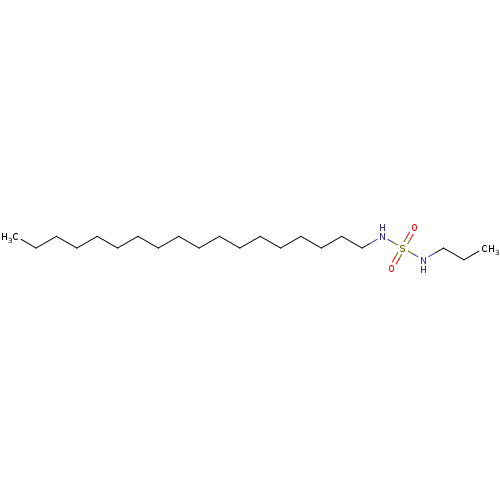 (CHEMBL219156 | N-octadecyl-N'-propylsulfamide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Activation of human PPARalpha by GST pull down assay | J Med Chem 50: 389-93 (2007) Article DOI: 10.1021/jm0601102 BindingDB Entry DOI: 10.7270/Q2HX1CB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50202585 (CHEMBL387094 | N-hexadecyl-N'-propylsulfamide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Activation of human PPARalpha by GST pull down assay | J Med Chem 50: 389-93 (2007) Article DOI: 10.1021/jm0601102 BindingDB Entry DOI: 10.7270/Q2HX1CB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50202584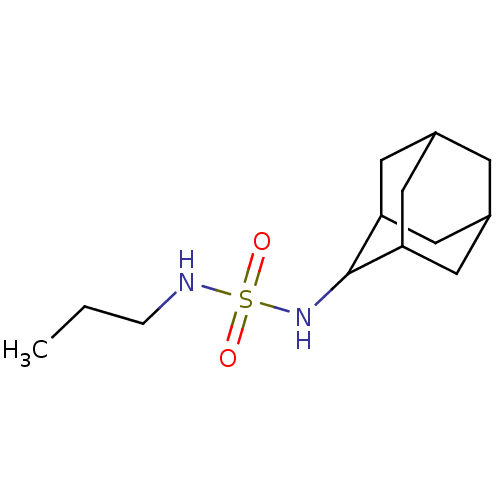 (CHEMBL218643 | N-(2-adamantyl)-N'-propylsulfamide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Activation of human PPARalpha by GST pull down assay | J Med Chem 50: 389-93 (2007) Article DOI: 10.1021/jm0601102 BindingDB Entry DOI: 10.7270/Q2HX1CB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM24566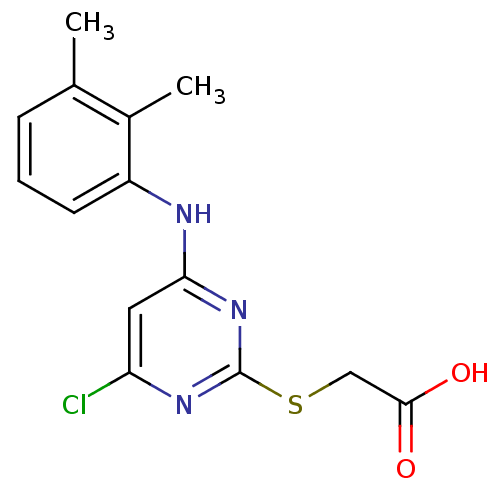 (2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 740 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Activation of human PPARalpha by GST pull down assay | J Med Chem 50: 389-93 (2007) Article DOI: 10.1021/jm0601102 BindingDB Entry DOI: 10.7270/Q2HX1CB0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292324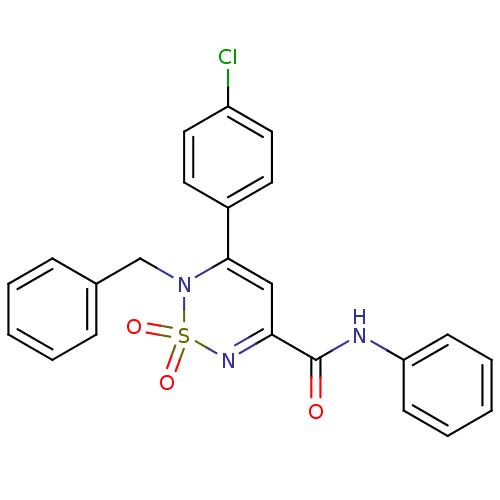 (CHEMBL393324 | N-phenyl-2-benzyl-3-(4-chlorophenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.73E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292325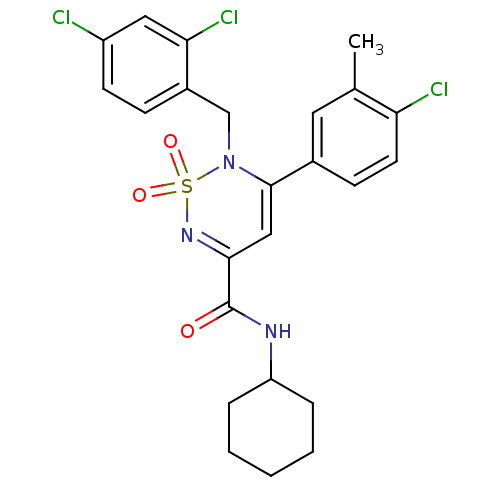 (CHEMBL240109 | N-cyclohexyl-2-(2,4-dichlorobenzyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.69E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292326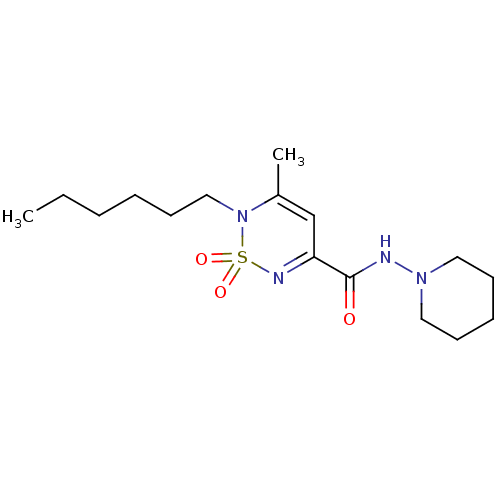 (CHEMBL239257 | N-(piperidin-1-yl)-2-hexyl-3-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.69E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292327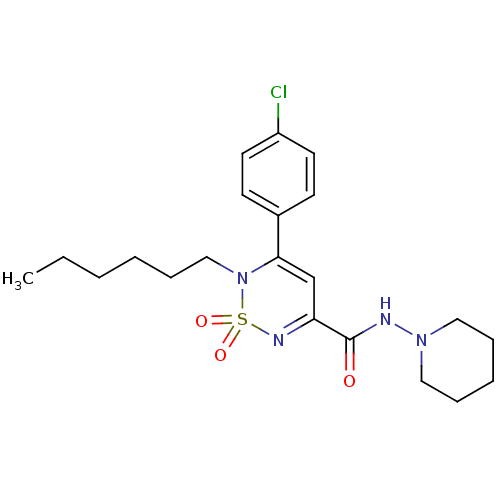 (CHEMBL241774 | N-(piperidin-1-yl)-3-(4-chloropheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.44E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292328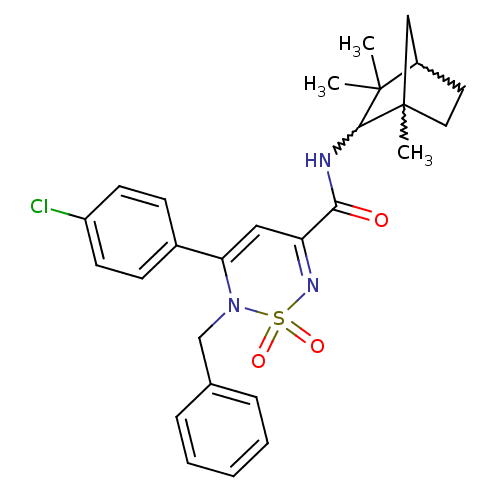 (CHEMBL241567 | N-(1,3,3-trimethylbicyclo[2.2.1]hep...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.99E+3 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292329 (CHEMBL240324 | N-(piperidin-1-yl)-2-benzyl-3-(4-ch...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50292330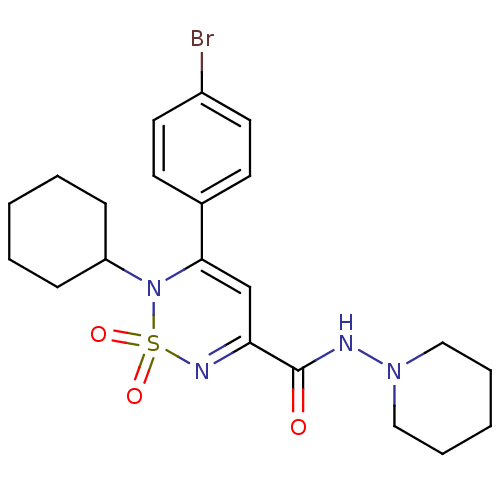 (CHEMBL391839 | N-(piperidin-1-yl)-3-(4-bromophenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in ICR mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 15: 7480-93 (2007) Article DOI: 10.1016/j.bmc.2007.07.056 BindingDB Entry DOI: 10.7270/Q2M04551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50202579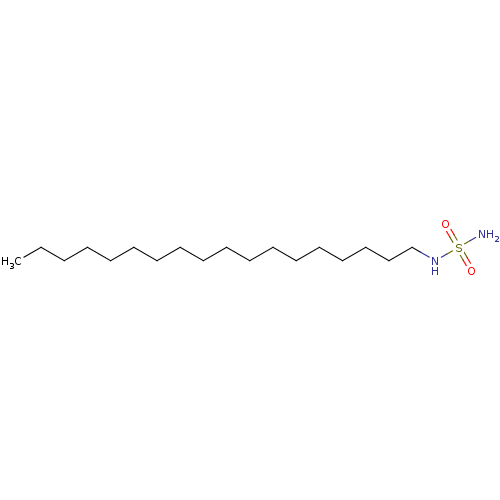 (CHEMBL218794 | N-octadecylsulfamide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Activation of human PPARalpha by GST pull down assay | J Med Chem 50: 389-93 (2007) Article DOI: 10.1021/jm0601102 BindingDB Entry DOI: 10.7270/Q2HX1CB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50202580 (CHEMBL374076 | N-oleyl-N'-propylsulfamide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Activation of human PPARalpha by GST pull down assay | J Med Chem 50: 389-93 (2007) Article DOI: 10.1021/jm0601102 BindingDB Entry DOI: 10.7270/Q2HX1CB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||