

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM9914 (3-[(R)-1H-imidazol-1-yl(4-nitrophenyl)methyl]-4H-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bologna | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 7282-9 (2005) Article DOI: 10.1021/jm058042r BindingDB Entry DOI: 10.7270/Q2JS9NN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9920 ((S)Fadrozole | 4-[(5S)-5,6,7,8-tetrahydroimidazo[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bologna | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 7282-9 (2005) Article DOI: 10.1021/jm058042r BindingDB Entry DOI: 10.7270/Q2JS9NN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191598 (3-((1H-imidazol-1-yl)methyl)-2-(4-nitrophenyl)-4H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191598 (3-((1H-imidazol-1-yl)methyl)-2-(4-nitrophenyl)-4H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9916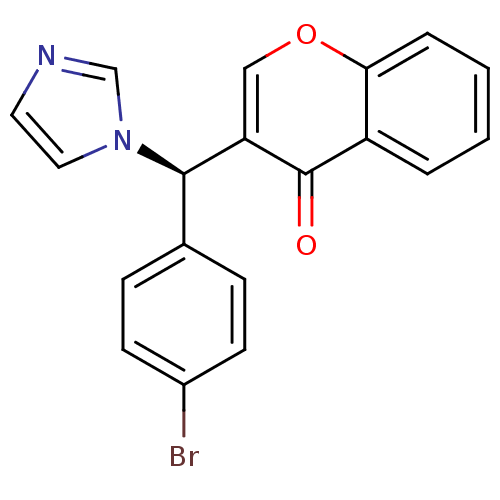 (3-[(R)-(4-bromophenyl)(1H-imidazol-1-yl)methyl]-4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bologna | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 7282-9 (2005) Article DOI: 10.1021/jm058042r BindingDB Entry DOI: 10.7270/Q2JS9NN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9475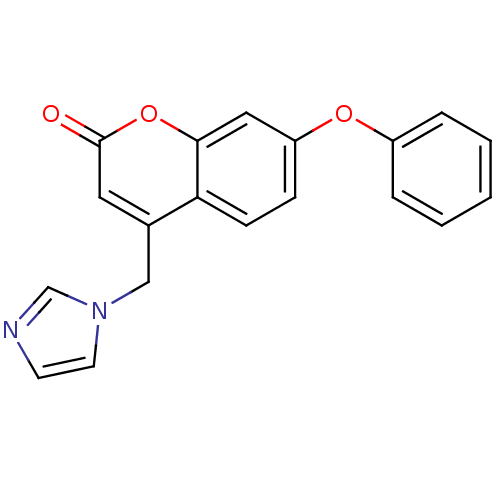 (4-(1H-Imidazol-1-ylmethyl)-7-phenoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611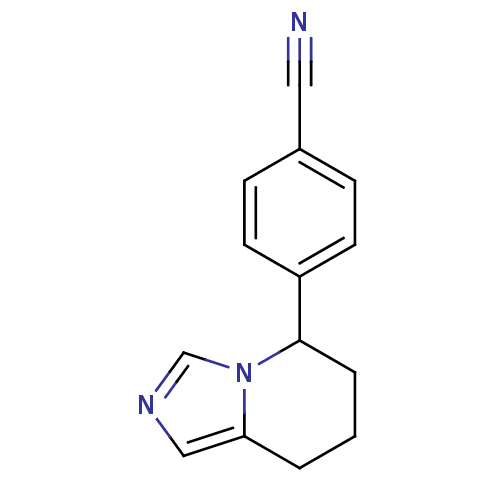 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191602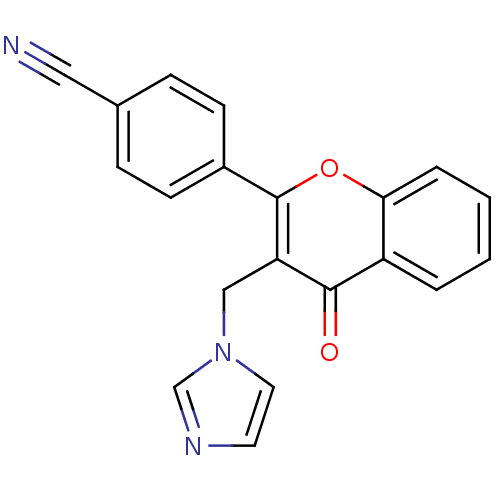 (4'-cyano-3-(imidazolylmethyl)flavone | 4-(3-((1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191602 (4'-cyano-3-(imidazolylmethyl)flavone | 4-(3-((1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191599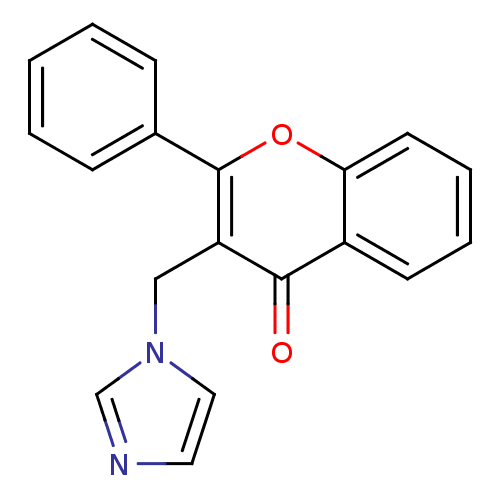 (3-((1H-imidazol-1-yl)methyl)-2-phenyl-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191599 (3-((1H-imidazol-1-yl)methyl)-2-phenyl-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9465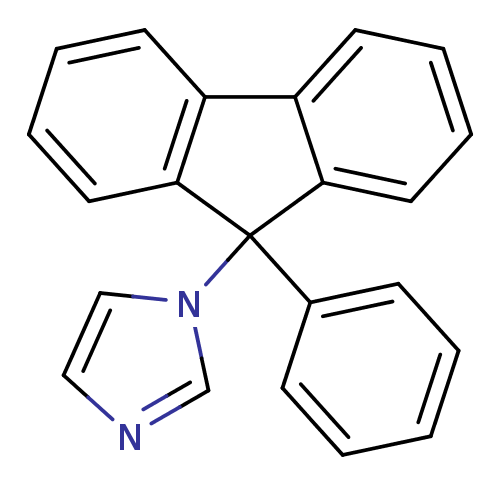 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-imidazole | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191600 (3-((1H-imidazol-1-yl)methyl)-2-(4-methoxyphenyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191600 (3-((1H-imidazol-1-yl)methyl)-2-(4-methoxyphenyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9915 (3-[(S)-1H-imidazol-1-yl(4-nitrophenyl)methyl]-4H-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bologna | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 7282-9 (2005) Article DOI: 10.1021/jm058042r BindingDB Entry DOI: 10.7270/Q2JS9NN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9483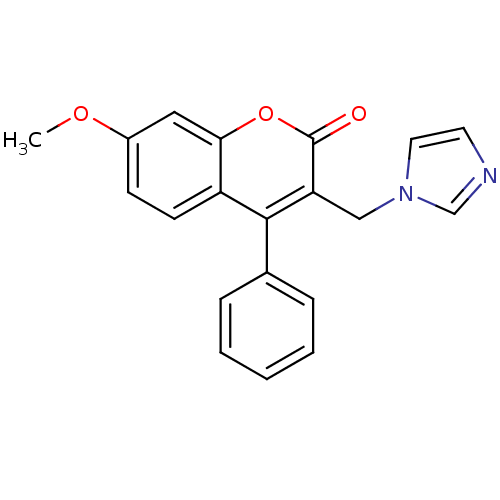 (3-(1H-Imidazol-1-ylmethyl)-7-methoxy-4-phenyl-2H-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8935 (5-[(6-methoxy-1-methyl-3,4-dihydronaphthalen-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9918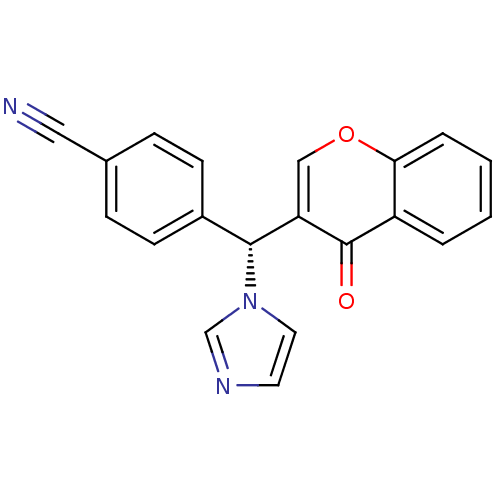 (4-[(R)-1H-imidazol-1-yl(4-oxo-4H-chromen-3-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bologna | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 7282-9 (2005) Article DOI: 10.1021/jm058042r BindingDB Entry DOI: 10.7270/Q2JS9NN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9477 (6-(1H-Imidazol-1-ylmethyl)-2H-chromen-2-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9485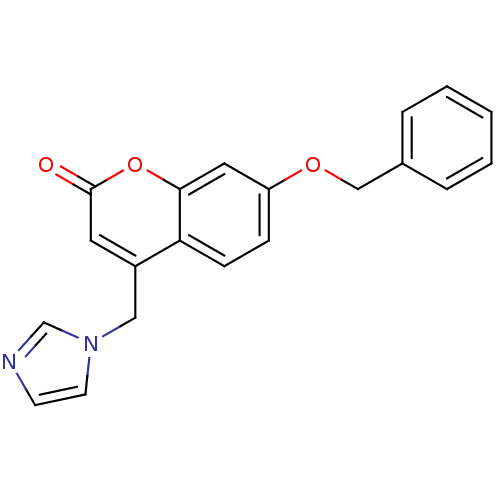 (7-(benzyloxy)-4-(1H-imidazol-1-ylmethyl)-2H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9476 (5-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9471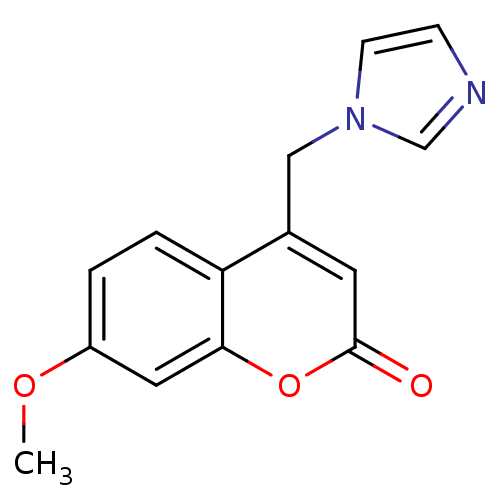 (4-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9917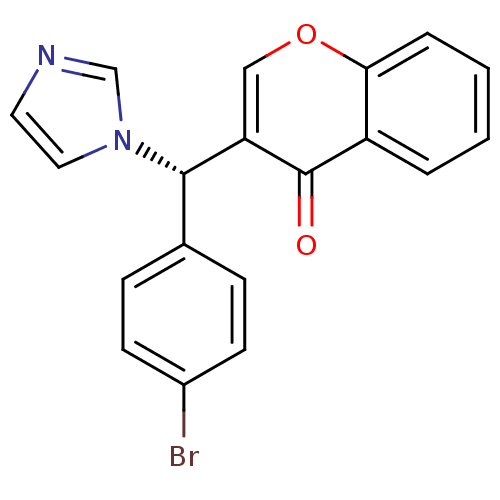 (3-[(S)-(4-bromophenyl)(1H-imidazol-1-yl)methyl]-4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bologna | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 7282-9 (2005) Article DOI: 10.1021/jm058042r BindingDB Entry DOI: 10.7270/Q2JS9NN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191597 (3-((1H-imidazol-1-yl)methyl)-2-(4-bromophenyl)-4H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191597 (3-((1H-imidazol-1-yl)methyl)-2-(4-bromophenyl)-4H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191603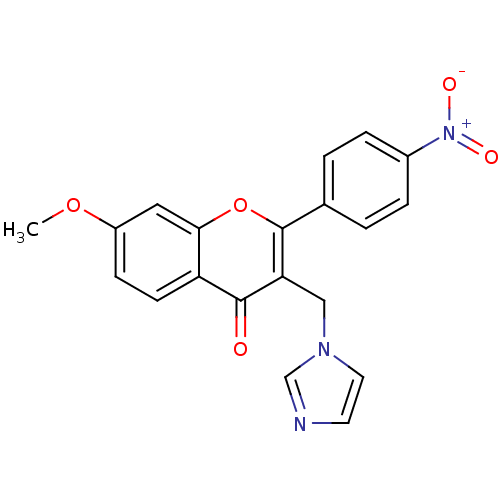 (3-((1H-imidazol-1-yl)methyl)-7-methoxy-2-(4-nitrop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191603 (3-((1H-imidazol-1-yl)methyl)-7-methoxy-2-(4-nitrop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50097372 (3-((1H-imidazol-1-yl)methyl)-7-methoxy-2-phenyl-4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50097372 (3-((1H-imidazol-1-yl)methyl)-7-methoxy-2-phenyl-4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9919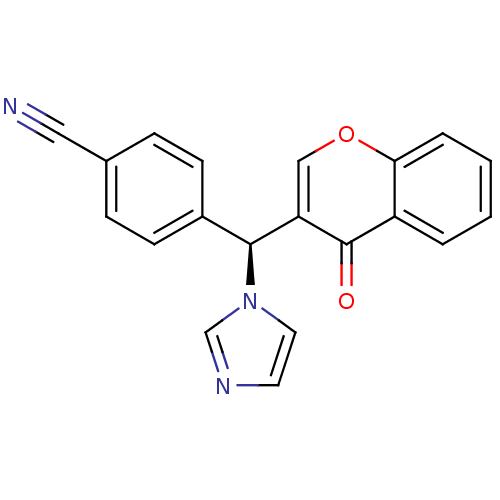 (4-[(S)-1H-imidazol-1-yl(4-oxo-4H-chromen-3-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bologna | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 7282-9 (2005) Article DOI: 10.1021/jm058042r BindingDB Entry DOI: 10.7270/Q2JS9NN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9478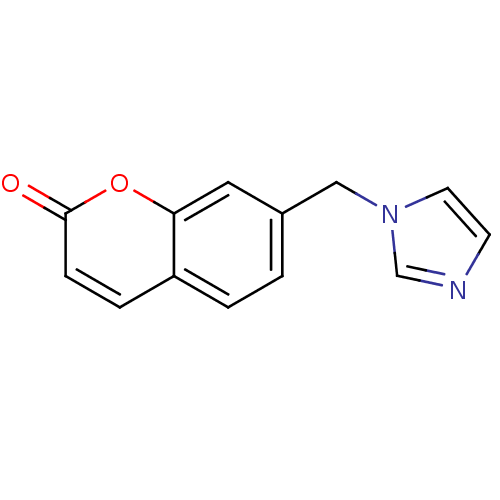 (7-(1H-Imidazol-1-ylmethyl)-2H-chromen-2-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9473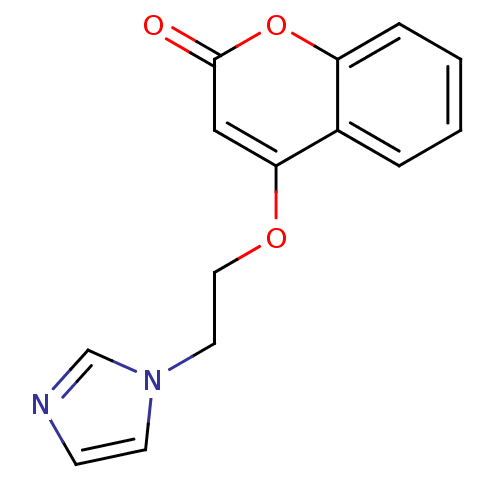 (4-[2-(1H-Imidazol-1-yl)ethoxy]-2H-chromen-2-one | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9486 (6-(1H-Imidazol-1-ylmethyl)-2H-chrome-2-thione | 6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191601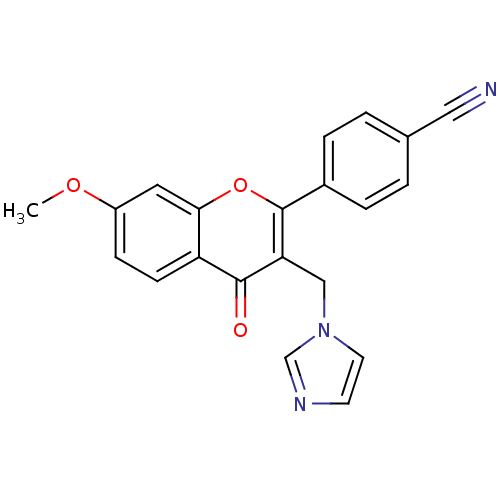 (4'-cyano-3-(imidazolylmethyl)-7-methoxyflavone | 4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191601 (4'-cyano-3-(imidazolylmethyl)-7-methoxyflavone | 4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9470 (4-(1H-Imidazol-1-ylmethyl)-2H-chromen-2-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9469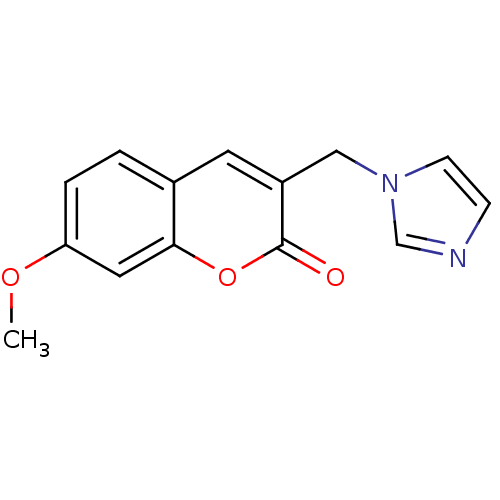 (3-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9462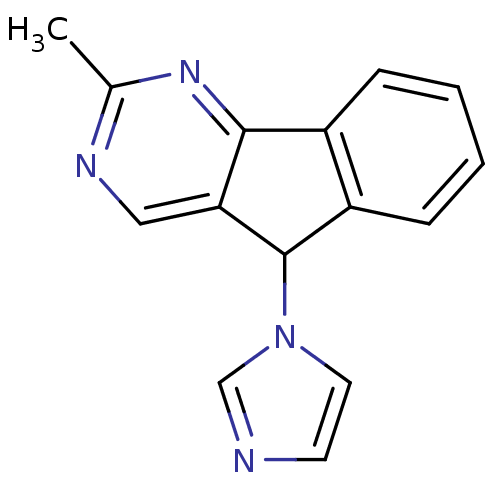 (1-{2-methyl-5H-indeno[1,2-d]pyrimidin-5-yl}-1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9464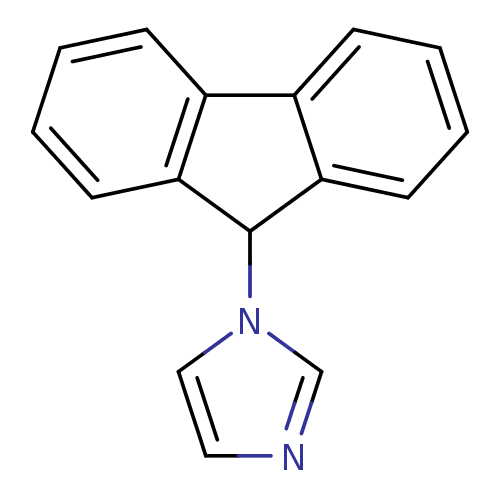 (1-(9H-Fluoren-9-yl)-1H-imidazole | CHEMBL225447 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9472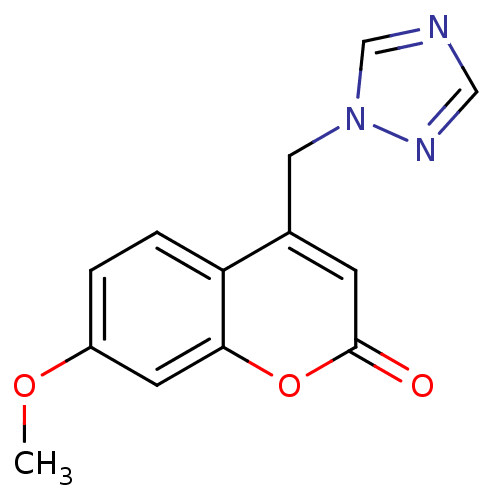 (7-Methoxy-4-(1H-1,2,4-triazol-1-ylmethyl)-2H-chrom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9468 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-1,2,4-triazole | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191596 (3-((1H-imidazol-1-yl)methyl)-2-(4-bromophenyl)-7-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50191596 (3-((1H-imidazol-1-yl)methyl)-2-(4-bromophenyl)-7-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of CYP19 | J Med Chem 49: 4777-80 (2006) Article DOI: 10.1021/jm060186y BindingDB Entry DOI: 10.7270/Q2668CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9482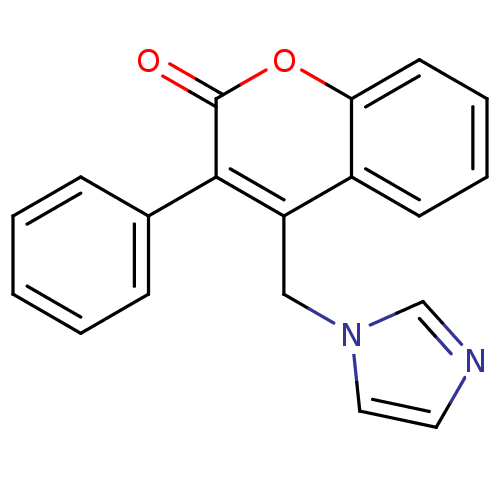 (4-(1H-Imidazol-1-ylmethyl)-3-phenyl-2H-chromen-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8935 (5-[(6-methoxy-1-methyl-3,4-dihydronaphthalen-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9463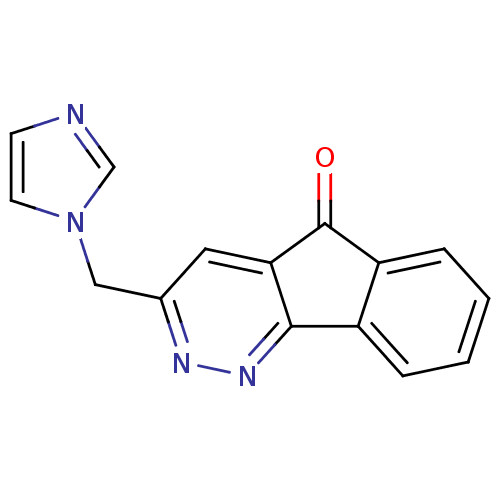 (3-(1H-Imidazol-1-ylmethyl)-5H-indeno[1,2-c]pyridaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9463 (3-(1H-Imidazol-1-ylmethyl)-5H-indeno[1,2-c]pyridaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9465 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-imidazole | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9466 (4-(9-Phenyl-9H-fluoren-9-yl)-4H-1,2,4-triazole | F...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9467 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-1,2,3-triazole | F...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 83 total ) | Next | Last >> |