

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474596 (CHEMBL79332) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474584 (CHEMBL79387) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474594 (CHEMBL86050) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474583 (CHEMBL86051) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474587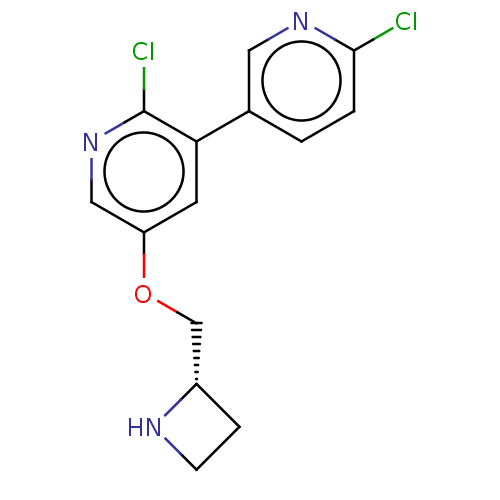 (CHEMBL314718) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474595 (CHEMBL79594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474582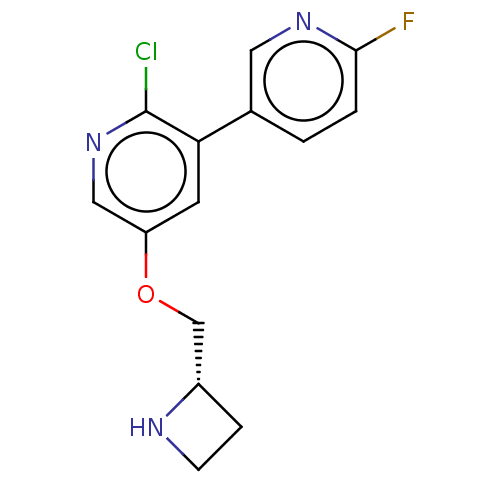 (CHEMBL83444) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474593 (CHEMBL313877) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474591 (CHEMBL79515) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474598 (CHEMBL79702) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474592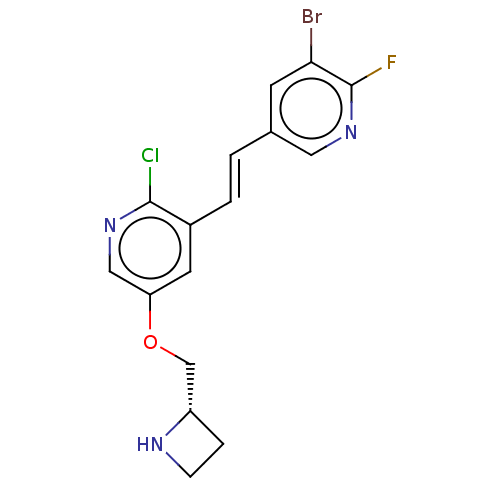 (CHEMBL83738) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474585 (CHEMBL312132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474590 (CHEMBL309292) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114773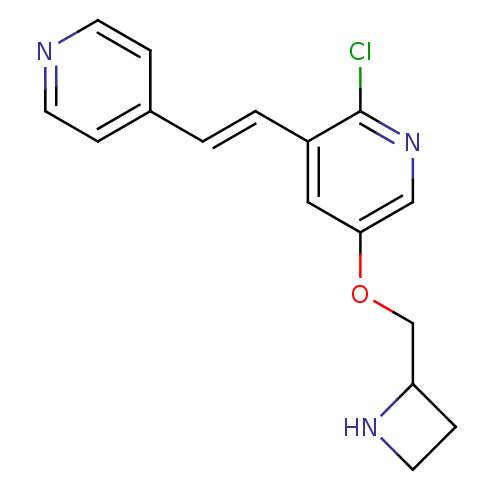 (5-(Azetidin-2-ylmethoxy)-2-chloro-3-(2-pyridin-4-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity for nAChR with [3H]-imidacloprid in Drosophila | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474586 (CHEMBL80040) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474588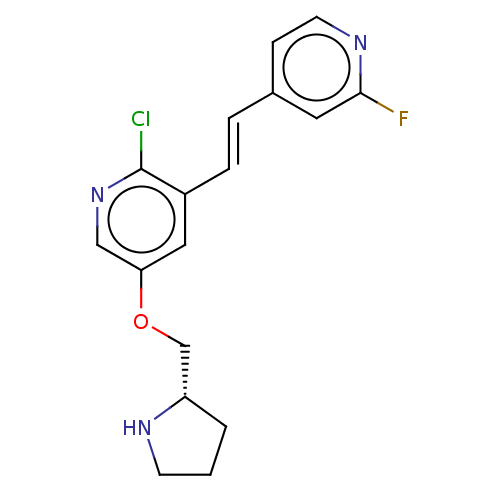 (CHEMBL310197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity for nAChR with [3H]-imidacloprid in Myzus persicae | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114771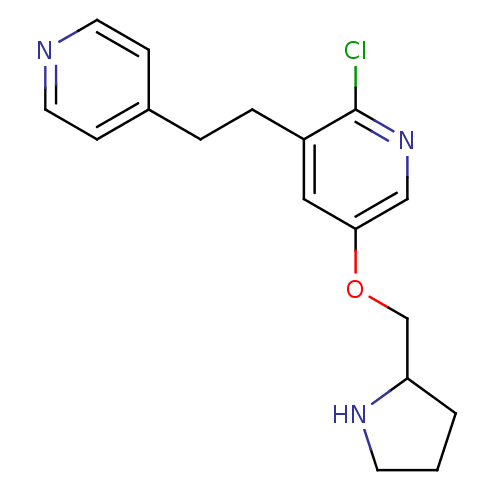 (2-Chloro-3-(2-pyridin-4-yl-ethyl)-5-(pyrrolidin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474597 (CHEMBL83608) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114774 (2-Chloro-3-(2-pyridin-4-yl-vinyl)-5-(pyrrolidin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity for nAChR with [3H]-imidacloprid in Drosophila | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474581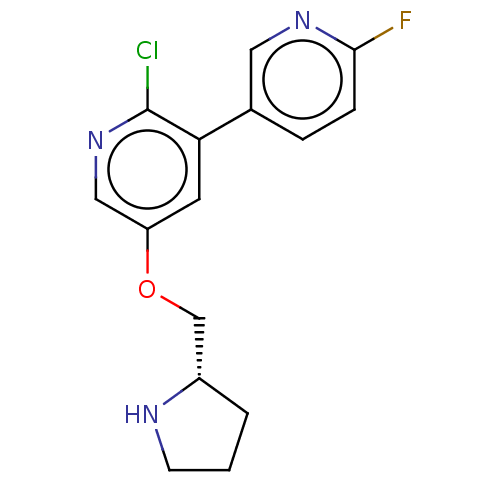 (CHEMBL83986) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474580 (CHEMBL84154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474599 (CHEMBL79737) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114768 (2-Chloro-5-(1-methyl-pyrrolidin-2-ylmethoxy)-3-(2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114767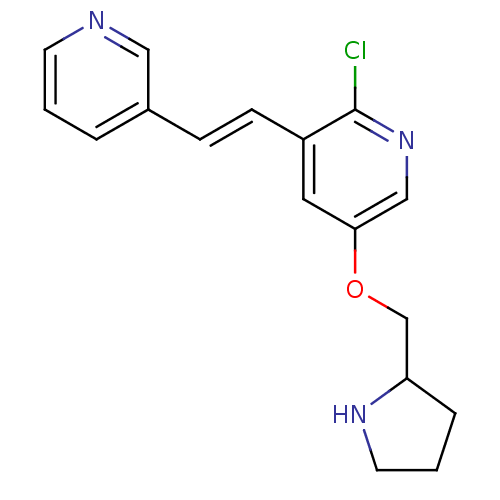 (2-Chloro-3-(2-pyridin-3-yl-vinyl)-5-(pyrrolidin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity for nAChR with [3H]-imidacloprid in Drosophila | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114769 (2-Chloro-5-(1-methyl-pyrrolidin-2-ylmethoxy)-3-(2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity for nAChR with [3H]-imidacloprid in Myzus persicae | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50062639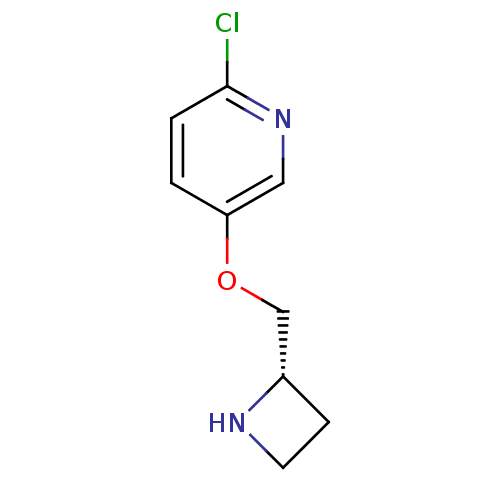 (5-((S)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50066788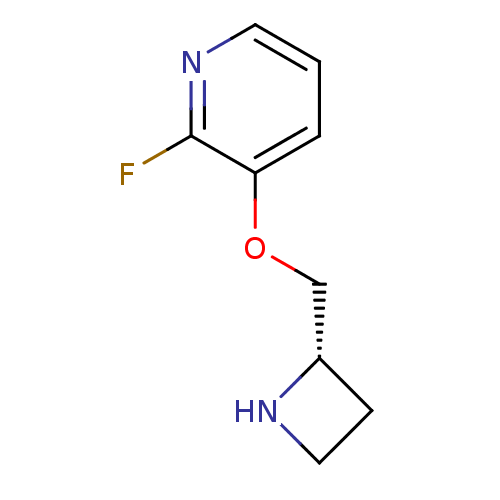 ((S)-3-(azetidin-2-ylmethoxy)-2-fluoropyridine | 3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474589 (CHEMBL79854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114775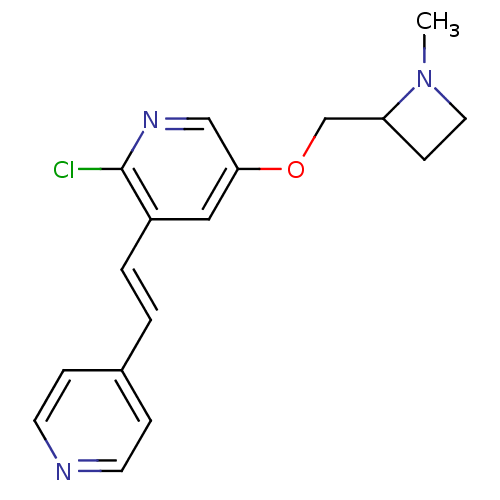 (2-Chloro-5-(1-methyl-azetidin-2-ylmethoxy)-3-(2-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114778 (2-Chloro-5-(1-methyl-pyrrolidin-2-ylmethoxy)-3-(2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity for nAChR with [3H]-imidacloprid in Myzus persicae | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114776 (2-Chloro-5-(1-methyl-pyrrolidin-2-ylmethoxy)-3-(2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity for nAChR with [3H]-imidacloprid in Myzus persicae | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50114777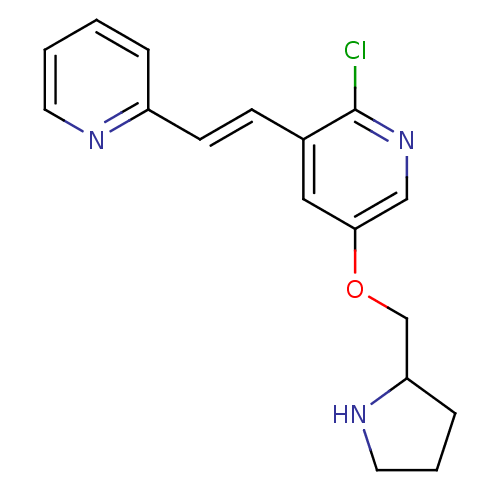 (2-Chloro-3-(2-pyridin-2-yl-vinyl)-5-(pyrrolidin-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity for nAChR with [3H]-imidacloprid in Drosophila | J Med Chem 45: 2841-9 (2002) BindingDB Entry DOI: 10.7270/Q2FN15JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21279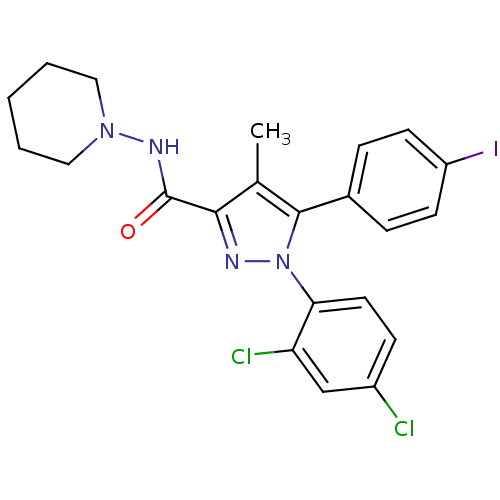 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172556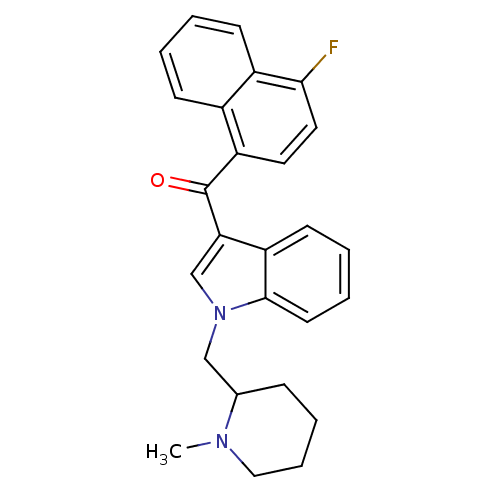 ((4-Fluoro-naphthalen-1-yl)-[1-(1-methyl-piperidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description In vitro binding affinity aganist rat cannabinoid receptor 1 using 0.5 nM [3H]-CP- 55940 | J Med Chem 48: 5813-22 (2005) Article DOI: 10.1021/jm0502743 BindingDB Entry DOI: 10.7270/Q23X865M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172553 (CHEMBL68641 | [1-(1-Methyl-piperidin-2-ylmethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description In vitro binding affinity aganist rat cannabinoid receptor 1 using 0.5 nM [3H]-CP- 55940 | J Med Chem 48: 5813-22 (2005) Article DOI: 10.1021/jm0502743 BindingDB Entry DOI: 10.7270/Q23X865M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172549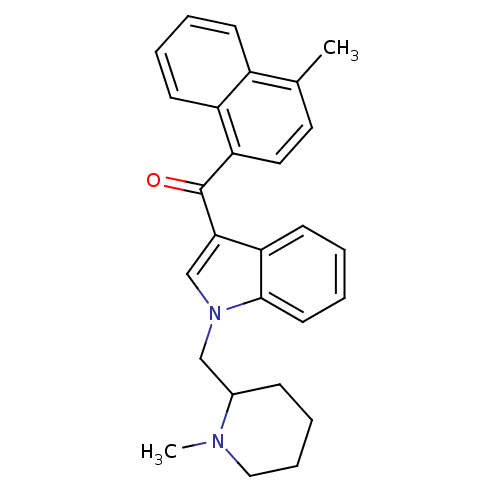 ((4-Methyl-naphthalen-1-yl)-[1-(1-methyl-piperidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description In vitro binding affinity aganist rat cannabinoid receptor 1 using 0.5 nM [3H]-CP- 55940 | J Med Chem 48: 5813-22 (2005) Article DOI: 10.1021/jm0502743 BindingDB Entry DOI: 10.7270/Q23X865M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172543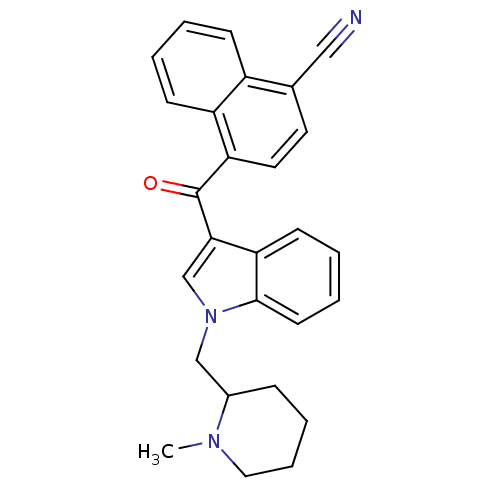 (4-[1-(1-Methyl-piperidin-2-ylmethyl)-1H-indole-3-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description In vitro binding affinity aganist rat cannabinoid receptor 1 using 0.5 nM [3H]-CP- 55940 | J Med Chem 48: 5813-22 (2005) Article DOI: 10.1021/jm0502743 BindingDB Entry DOI: 10.7270/Q23X865M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172545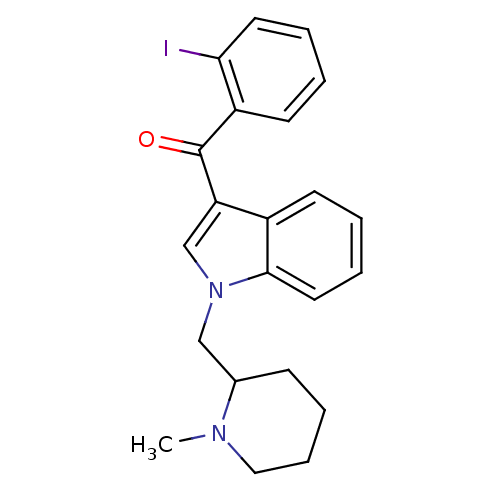 ((2-Iodo-phenyl)-[1-((S)-1-methyl-piperidin-2-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description In vitro binding affinity aganist rat cannabinoid receptor 1 using 0.5 nM [3H]-CP- 55940 | J Med Chem 48: 5813-22 (2005) Article DOI: 10.1021/jm0502743 BindingDB Entry DOI: 10.7270/Q23X865M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172555 (CHEMBL364223 | [4-(2-Hydroxy-ethyl)-naphthalen-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description In vitro binding affinity aganist rat cannabinoid receptor 1 using 0.5 nM [3H]-CP- 55940 | J Med Chem 48: 5813-22 (2005) Article DOI: 10.1021/jm0502743 BindingDB Entry DOI: 10.7270/Q23X865M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172546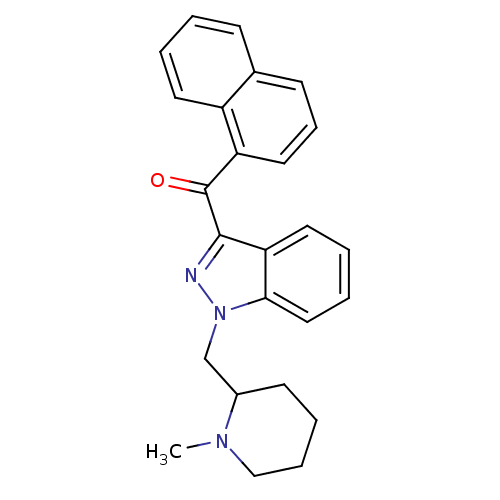 (CHEMBL194313 | [1-(1-Methyl-piperidin-2-ylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description In vitro binding affinity aganist rat cannabinoid receptor 1 using 0.5 nM [3H]-CP- 55940 | J Med Chem 48: 5813-22 (2005) Article DOI: 10.1021/jm0502743 BindingDB Entry DOI: 10.7270/Q23X865M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123685 (4-Bromo-5-(4-chloro-phenyl)-1-(2,4-dichloro-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172551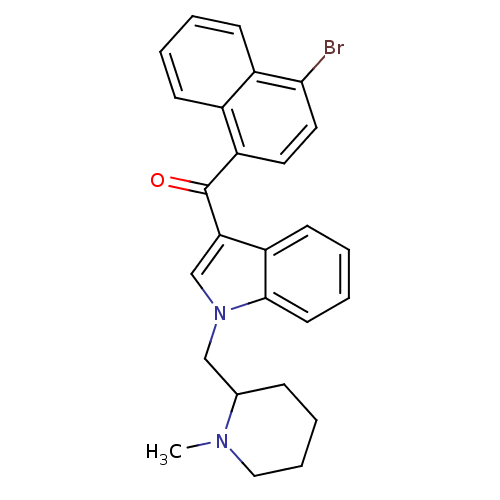 ((4-Bromo-naphthalen-1-yl)-[1-(1-methyl-piperidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description In vitro binding affinity aganist rat cannabinoid receptor 1 using 0.5 nM [3H]-CP- 55940 | J Med Chem 48: 5813-22 (2005) Article DOI: 10.1021/jm0502743 BindingDB Entry DOI: 10.7270/Q23X865M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172547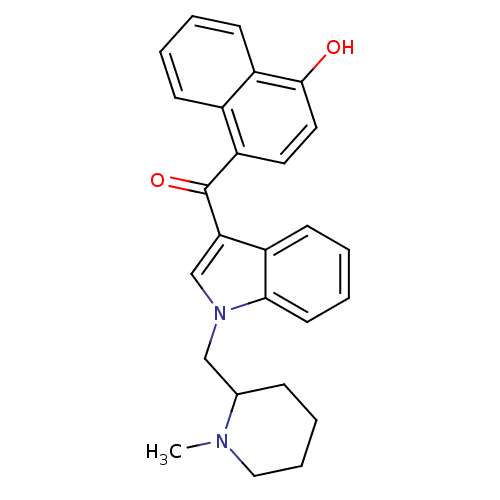 ((4-Hydroxy-naphthalen-1-yl)-[1-(1-methyl-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description In vitro binding affinity aganist rat cannabinoid receptor 1 using 0.5 nM [3H]-CP- 55940 | J Med Chem 48: 5813-22 (2005) Article DOI: 10.1021/jm0502743 BindingDB Entry DOI: 10.7270/Q23X865M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172548 ((4-Methoxy-naphthalen-1-yl)-[1-(1-methyl-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description In vitro binding affinity aganist rat cannabinoid receptor 1 using 0.5 nM [3H]-CP- 55940 | J Med Chem 48: 5813-22 (2005) Article DOI: 10.1021/jm0502743 BindingDB Entry DOI: 10.7270/Q23X865M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50172544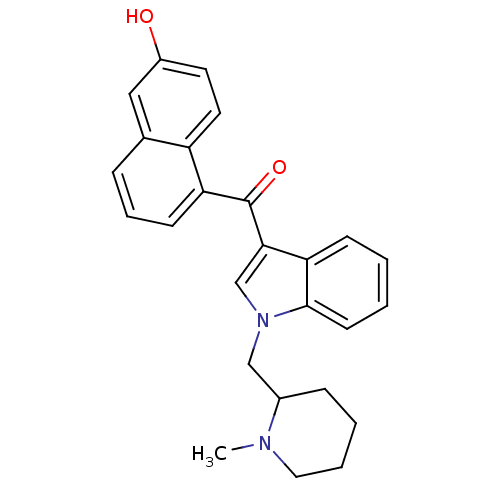 ((6-Hydroxy-naphthalen-1-yl)-[1-(1-methyl-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description In vitro binding affinity aganist rat cannabinoid receptor 1 using 0.5 nM [3H]-CP- 55940 | J Med Chem 48: 5813-22 (2005) Article DOI: 10.1021/jm0502743 BindingDB Entry DOI: 10.7270/Q23X865M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123690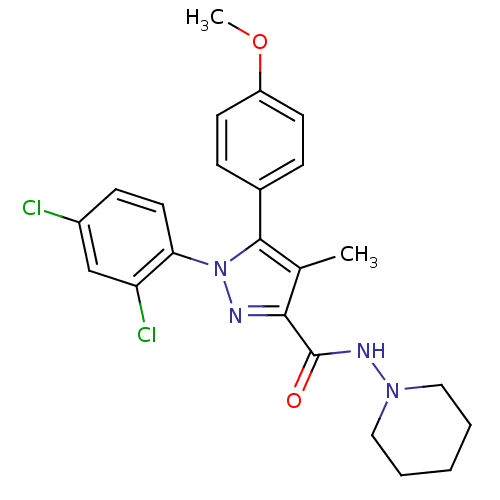 (1-(2,4-Dichloro-phenyl)-5-(4-methoxy-phenyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123688 (4-Bromo-1-(2,4-dichloro-phenyl)-5-(4-methoxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |