

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kynureninase (Homo sapiens (Human)) | BDBM50109155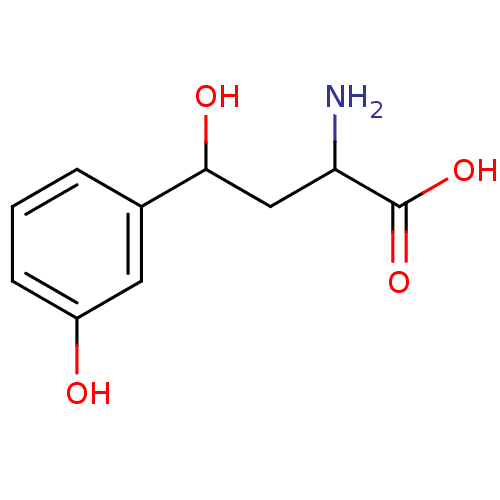 (2-Amino-4-hydroxy-4-(3-hydroxy-phenyl)-butyric aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Inhibition of human kynureninase | J Med Chem 52: 389-96 (2009) Article DOI: 10.1021/jm8010806 BindingDB Entry DOI: 10.7270/Q2DZ0860 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatic-L-amino-acid decarboxylase (Sus scrofa) | BDBM50005740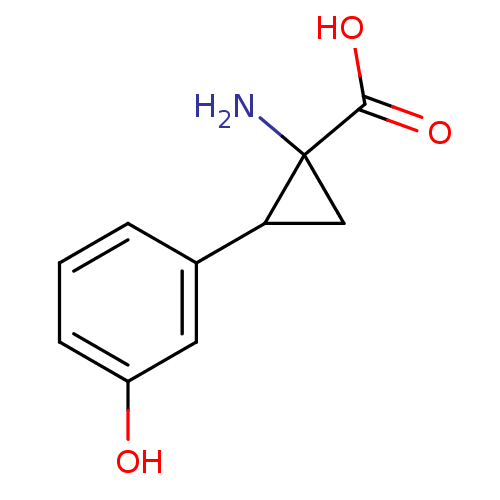 ((+)-1-Amino-2-(3-hydroxy-phenyl)-cyclopropa necarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Inhibitory activity of the compound against pig kidney L-aromatic amino acid decarboxylase (Dopamine decarboxylase) | J Med Chem 35: 1410-7 (1992) BindingDB Entry DOI: 10.7270/Q2W094WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatic-L-amino-acid decarboxylase (Sus scrofa) | BDBM50005743 ((-)-1-Amino-2-(3-hydroxy-phenyl)-cyclopropanecarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Inhibitory activity of the compound against pig kidney L-aromatic amino acid decarboxylase(Dopa decarboxylase) | J Med Chem 35: 1410-7 (1992) BindingDB Entry DOI: 10.7270/Q2W094WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynureninase (Homo sapiens (Human)) | BDBM50265459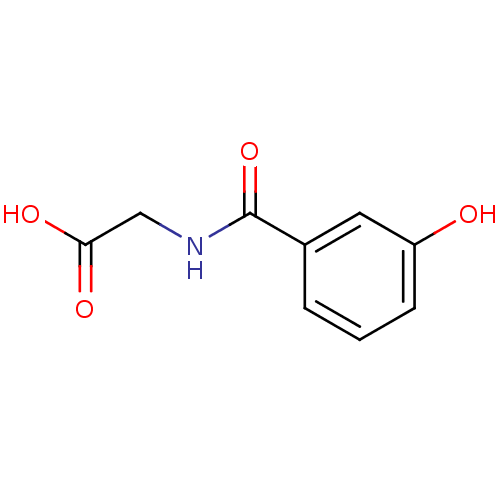 (3-Hydroxyhippuric acid | CHEMBL447627) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Inhibition of human kynureninase | J Med Chem 52: 389-96 (2009) Article DOI: 10.1021/jm8010806 BindingDB Entry DOI: 10.7270/Q2DZ0860 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatic-L-amino-acid decarboxylase (Sus scrofa) | BDBM50005741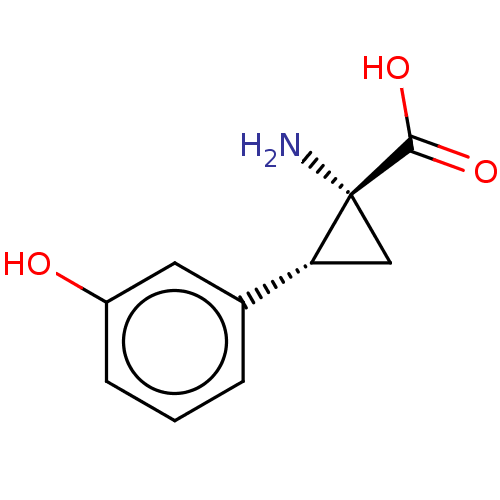 ((+)-1-Amino-2-(3-hydroxy-phenyl)-cyclopropanecarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Inhibitory activity of the compound against pig kidney L-aromatic amino acid decarboxylase (Dopa decarboxylase) | J Med Chem 35: 1410-7 (1992) BindingDB Entry DOI: 10.7270/Q2W094WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatic-L-amino-acid decarboxylase (Sus scrofa) | BDBM50005740 ((+)-1-Amino-2-(3-hydroxy-phenyl)-cyclopropa necarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Inhibitory activity of the compound against pig kidney L-aromatic amino acid decarboxylase(Dopa decarboxylase) | J Med Chem 35: 1410-7 (1992) BindingDB Entry DOI: 10.7270/Q2W094WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatic-L-amino-acid decarboxylase (Sus scrofa) | BDBM50005742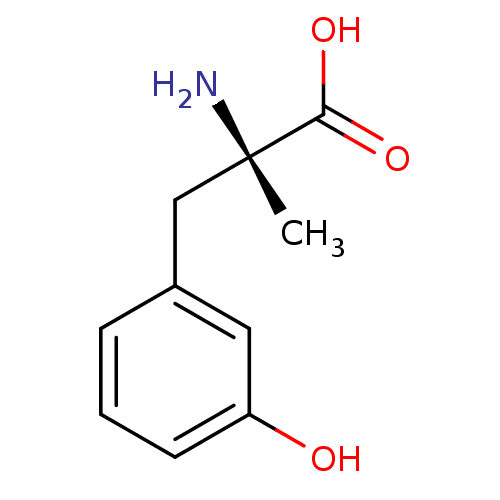 ((+)-D-m-Tyrosine2-Amino-3-(3-hydroxy-phenyl)-2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Inhibitory activity of the compound against pig kidney L-aromatic amino acid decarboxylase (Dopamine decarboxylase) | J Med Chem 35: 1410-7 (1992) BindingDB Entry DOI: 10.7270/Q2W094WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50061916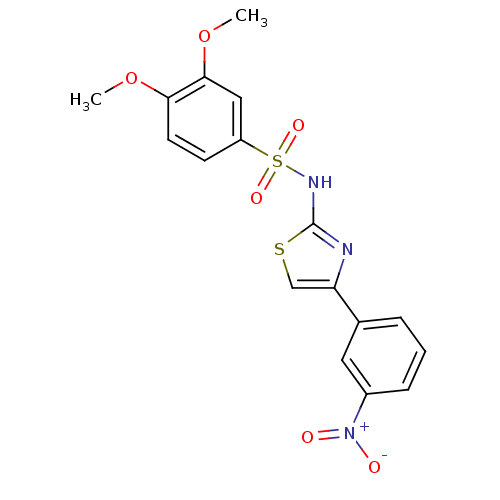 (3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Inhibition of KMO (unknown origin) | Bioorg Med Chem Lett 27: 1705-1708 (2017) Article DOI: 10.1016/j.bmcl.2017.02.080 BindingDB Entry DOI: 10.7270/Q2154K97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||