

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50388546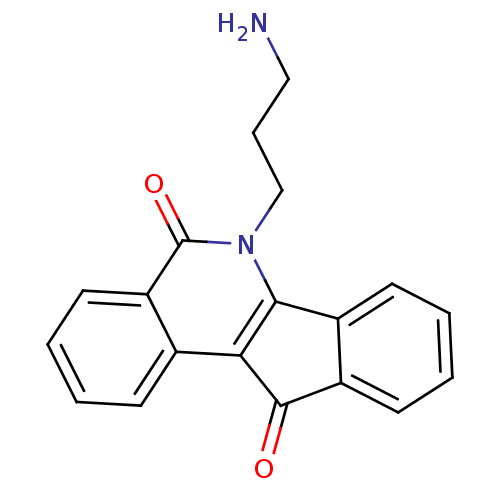 (CHEMBL213072 | CHEMBL333363) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant Tdp1 after 1 hr by FRET assay | J Med Chem 55: 4457-78 (2012) Article DOI: 10.1021/jm300335n BindingDB Entry DOI: 10.7270/Q2SB46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM412676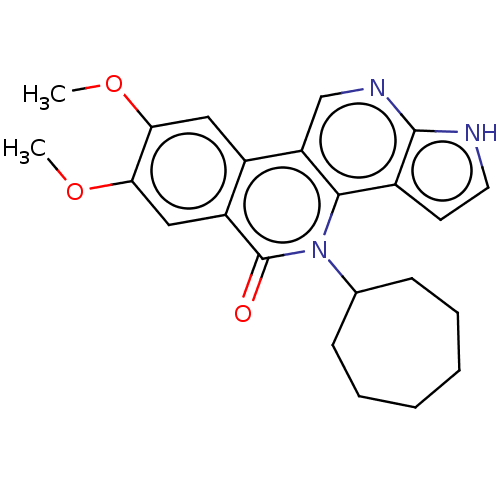 (US10399979, Compound 17c) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM412680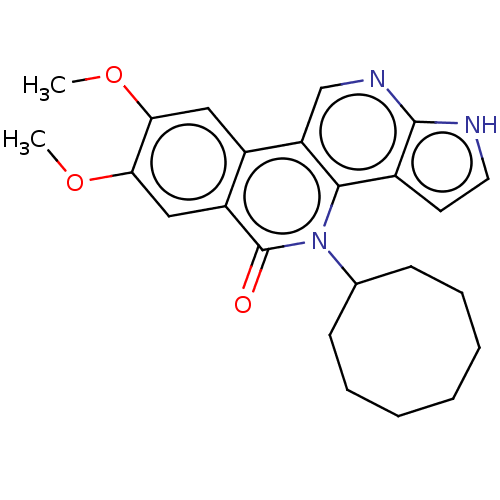 (US10399979, Compound 17d) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM412676 (US10399979, Compound 17c) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM412674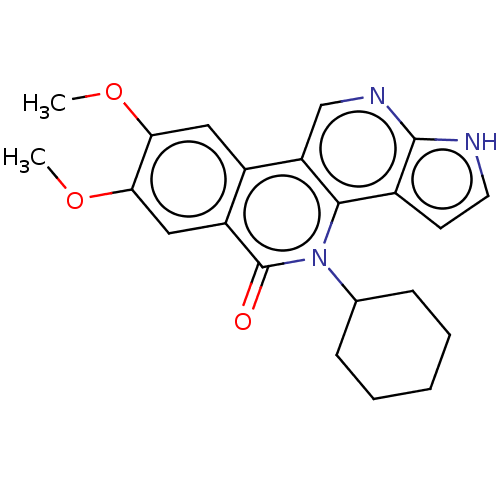 (US10399979, Compound 17a) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM412687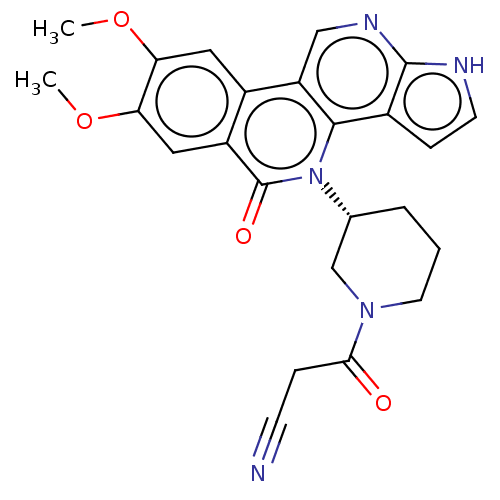 (US10399979, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM412675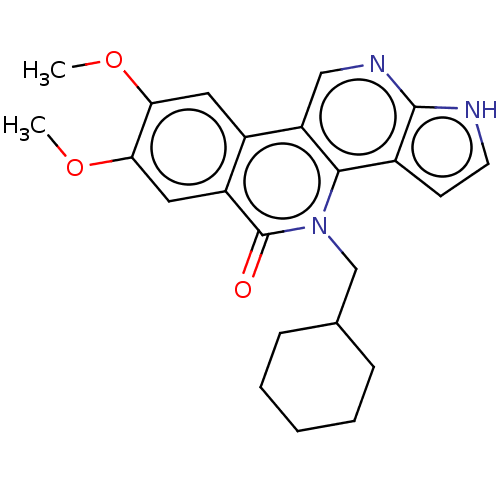 (US10399979, Compound 17b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM412680 (US10399979, Compound 17d) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM412674 (US10399979, Compound 17a) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM412673 (US10399979, Compound 13c) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM412676 (US10399979, Compound 17c) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM412687 (US10399979, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM412674 (US10399979, Compound 17a) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM412687 (US10399979, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM412690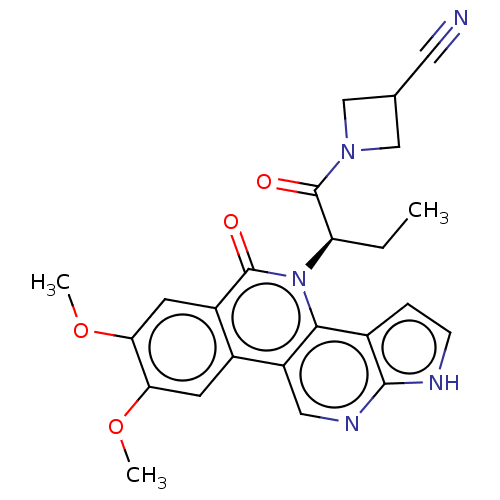 (US10399979, Compound 20a) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM412675 (US10399979, Compound 17b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM437709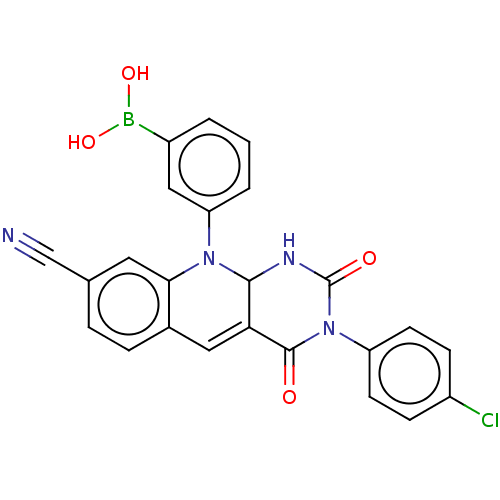 (US10617706, Example 143) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.85 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENTS OF THE UNIVERSITY OF MINNESOTA; THE USA, AS REPRESENTED BY THE SECRETARY, DEPT. OF HEALTH AND HUMAN SERVICES US Patent | Assay Description The enzymatic activity of TDP2 was measured with a SUMO hTDP2cat fluorescence-based biochemical assay. To a black 384-well plate, 10 μL of compo... | US Patent US10617706 (2020) BindingDB Entry DOI: 10.7270/Q20C4ZT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482161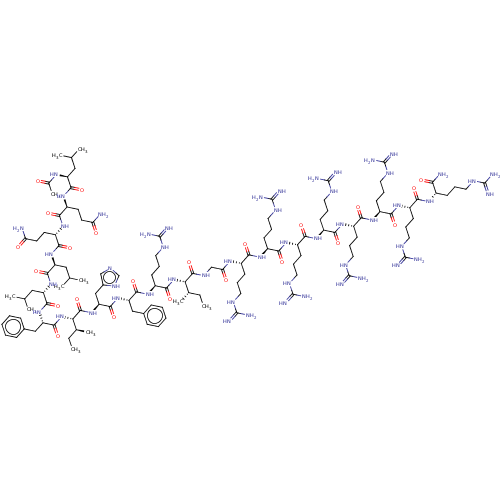 (CHEMBL1082256) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM412674 (US10399979, Compound 17a) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM412687 (US10399979, Compound 18) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482160 (CHEMBL1082257) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM437686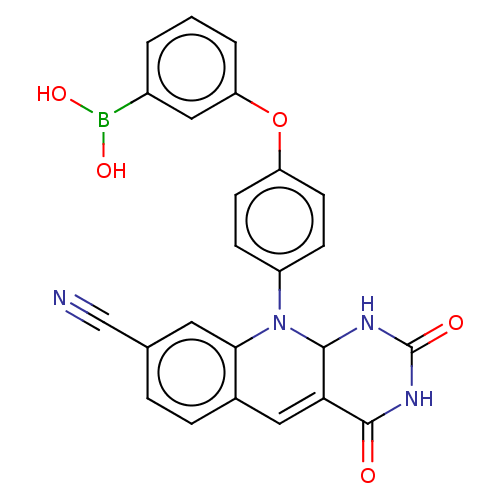 (US10617706, Example 120) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENTS OF THE UNIVERSITY OF MINNESOTA; THE USA, AS REPRESENTED BY THE SECRETARY, DEPT. OF HEALTH AND HUMAN SERVICES US Patent | Assay Description The enzymatic activity of TDP2 was measured with a SUMO hTDP2cat fluorescence-based biochemical assay. To a black 384-well plate, 10 μL of compo... | US Patent US10617706 (2020) BindingDB Entry DOI: 10.7270/Q20C4ZT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50466000 (CHEMBL4287893) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27135 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
St. John's University Curated by ChEMBL | Assay Description Inhibition of PARP-1 (unknown origin) using biotinylated NAD+ as substrate after 60 mins by spectrophotometry | J Med Chem 57: 5579-601 (2014) Article DOI: 10.1021/jm5002502 BindingDB Entry DOI: 10.7270/Q28C9XSS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482160 (CHEMBL1082257) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-end processing activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM437707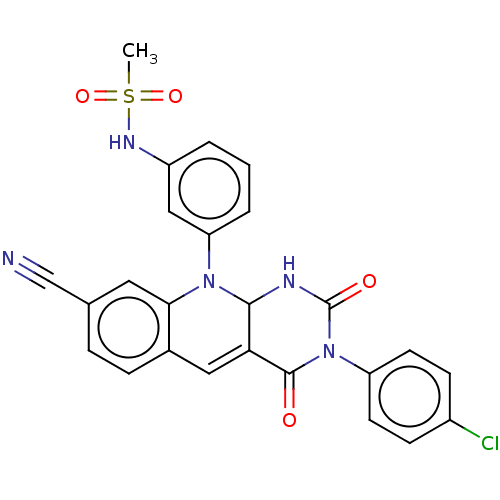 (US10617706, Example 141) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.09 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENTS OF THE UNIVERSITY OF MINNESOTA; THE USA, AS REPRESENTED BY THE SECRETARY, DEPT. OF HEALTH AND HUMAN SERVICES US Patent | Assay Description The enzymatic activity of TDP2 was measured with a SUMO hTDP2cat fluorescence-based biochemical assay. To a black 384-well plate, 10 μL of compo... | US Patent US10617706 (2020) BindingDB Entry DOI: 10.7270/Q20C4ZT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521692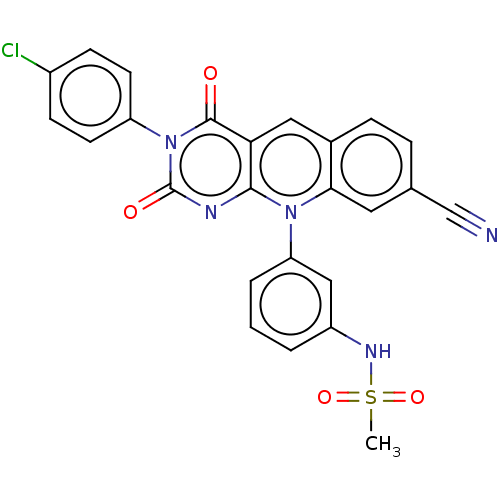 (CHEMBL4441013) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM437708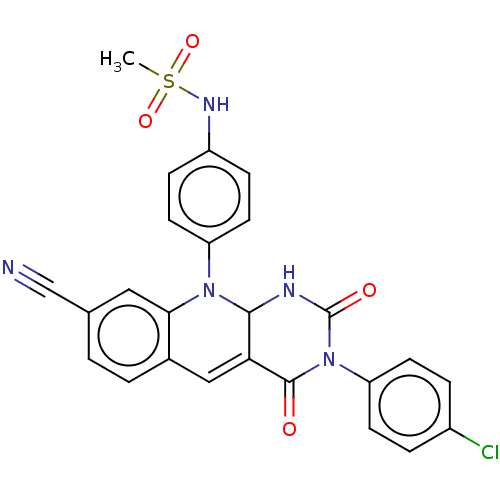 (US10617706, Example 142) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.49 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENTS OF THE UNIVERSITY OF MINNESOTA; THE USA, AS REPRESENTED BY THE SECRETARY, DEPT. OF HEALTH AND HUMAN SERVICES US Patent | Assay Description The enzymatic activity of TDP2 was measured with a SUMO hTDP2cat fluorescence-based biochemical assay. To a black 384-well plate, 10 μL of compo... | US Patent US10617706 (2020) BindingDB Entry DOI: 10.7270/Q20C4ZT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521699 (CHEMBL4572376) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM412676 (US10399979, Compound 17c) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM412672 (US10399979, Compound 13b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27566 (4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
St. John's University Curated by ChEMBL | Assay Description Inhibition of PARP-1 (unknown origin) using biotinylated NAD+ as substrate after 60 mins by spectrophotometry | J Med Chem 57: 5579-601 (2014) Article DOI: 10.1021/jm5002502 BindingDB Entry DOI: 10.7270/Q28C9XSS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase strand transfer activity by gel-based assay | J Med Chem 58: 1915-28 (2015) Article DOI: 10.1021/jm501799k BindingDB Entry DOI: 10.7270/Q2QJ7JZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM25351 (N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of recombinant HIV1 integrase using 5'-end-labeled 21-mer double-stranded DNA as substrate after 60 mins by el... | J Med Chem 56: 8588-98 (2013) Article DOI: 10.1021/jm401040b BindingDB Entry DOI: 10.7270/Q2BZ690C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM437703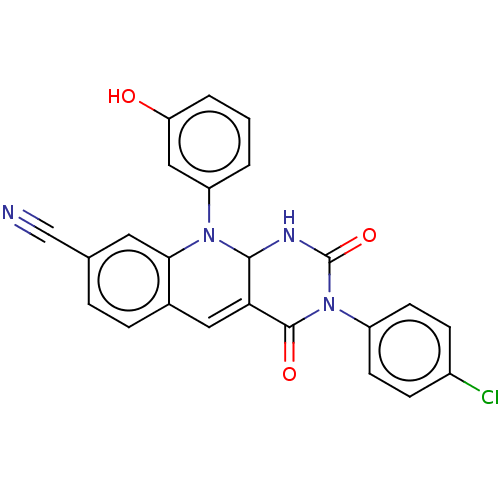 (US10617706, Example 137) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.25 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENTS OF THE UNIVERSITY OF MINNESOTA; THE USA, AS REPRESENTED BY THE SECRETARY, DEPT. OF HEALTH AND HUMAN SERVICES US Patent | Assay Description The enzymatic activity of TDP2 was measured with a SUMO hTDP2cat fluorescence-based biochemical assay. To a black 384-well plate, 10 μL of compo... | US Patent US10617706 (2020) BindingDB Entry DOI: 10.7270/Q20C4ZT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521689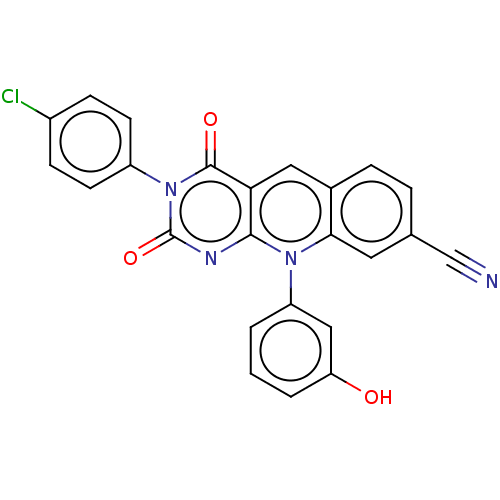 (CHEMBL4436202) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482161 (CHEMBL1082256) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-end processing activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020284 (CHEMBL3288833) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM437685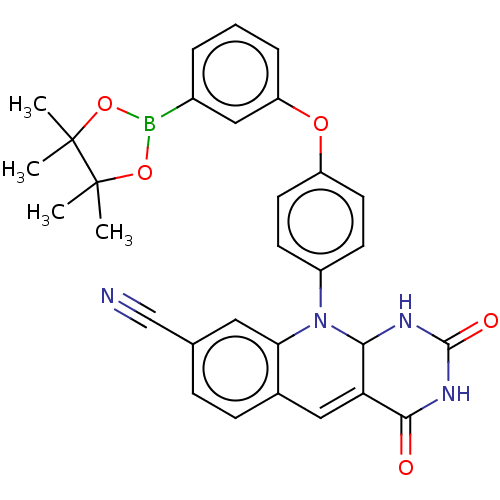 (US10617706, Example 119) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.28 | n/a | n/a | n/a | n/a | n/a | n/a |
REGENTS OF THE UNIVERSITY OF MINNESOTA; THE USA, AS REPRESENTED BY THE SECRETARY, DEPT. OF HEALTH AND HUMAN SERVICES US Patent | Assay Description The enzymatic activity of TDP2 was measured with a SUMO hTDP2cat fluorescence-based biochemical assay. To a black 384-well plate, 10 μL of compo... | US Patent US10617706 (2020) BindingDB Entry DOI: 10.7270/Q20C4ZT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020294 (CHEMBL3288834) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM412680 (US10399979, Compound 17d) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of TYK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50466000 (CHEMBL4287893) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM412671 (US10399979, Compound 13a) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM412672 (US10399979, Compound 13b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin) preincubated for 20 mins followed by [33P]-ATP addition measured after 2 hrs by filter-binding method | J Med Chem 61: 10440-10462 (2018) Article DOI: 10.1021/acs.jmedchem.8b00510 BindingDB Entry DOI: 10.7270/Q2BR8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020283 (CHEMBL3288832) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50020278 (CHEMBL3288827) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase using [gamma-32P]-labeled DNA as substrate | J Med Chem 57: 5190-202 (2014) Article DOI: 10.1021/jm5001908 BindingDB Entry DOI: 10.7270/Q2JS9S0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482699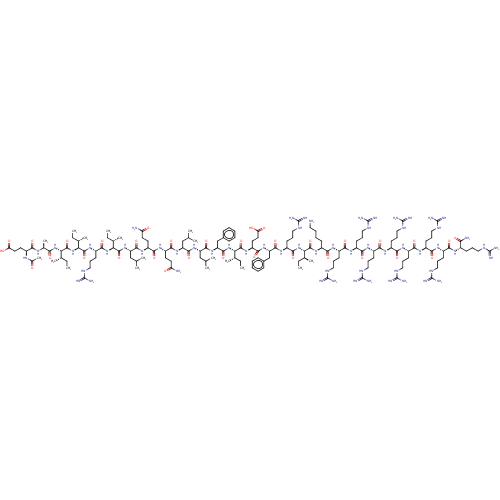 (CHEMBL1241174) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity expressed in Escherichia coli after 60 mins | Bioorg Med Chem 18: 6771-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.050 BindingDB Entry DOI: 10.7270/Q23N2661 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3118 total ) | Next | Last >> |