

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 1/2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against cyclooxygenase of human platelets was determined | J Med Chem 30: 726-9 (1987) BindingDB Entry DOI: 10.7270/Q2Q81G93 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004182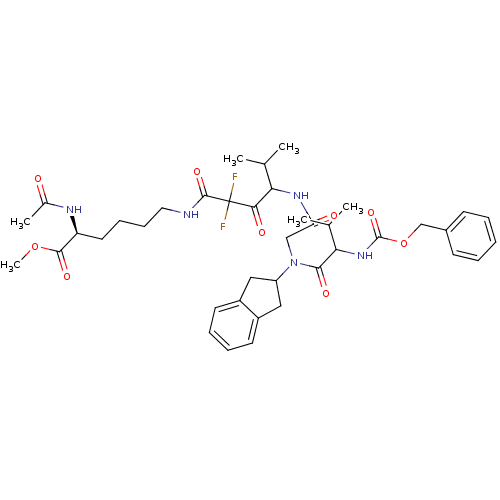 (2-Acetylamino-6-(4-{2-[(2-benzyloxycarbonylamino-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50070289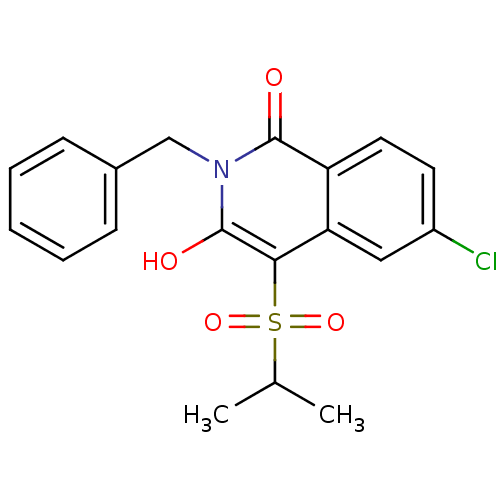 (2-Benzyl-6-chloro-3-hydroxy-4-(propane-2-sulfonyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004192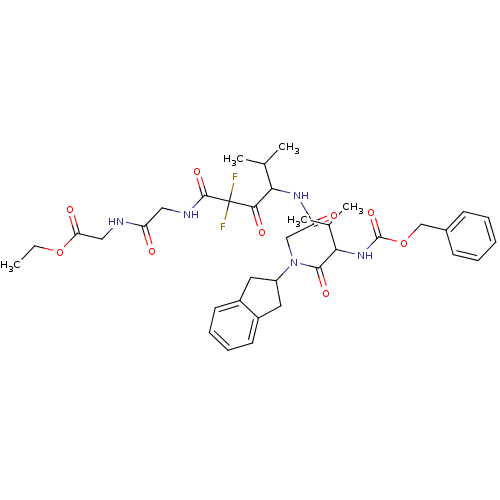 (CHEMBL344981 | [2-(4-{2-[(2-Benzyloxycarbonylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004184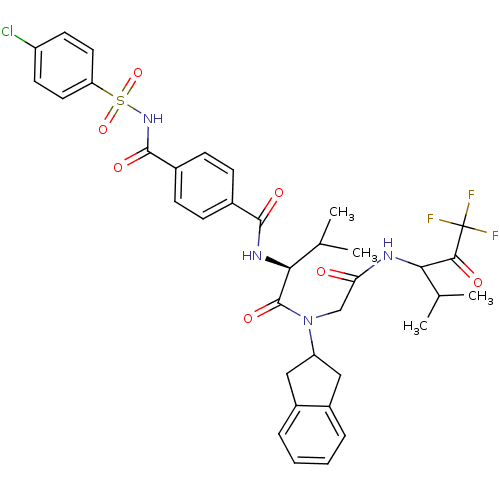 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-((S)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50070287 (2-(4-Fluoro-benzyl)-3-hydroxy-4-(propane-2-sulfony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production; Imax=84% | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50070291 (6-Chloro-2-(3,4-difluoro-benzyl)-3-hydroxy-4-(prop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004190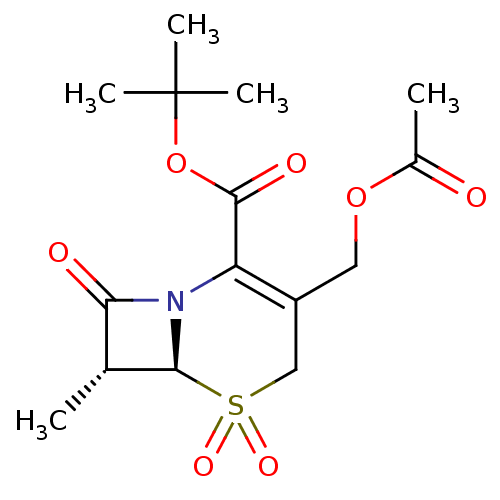 (3-Acetoxymethyl-7-methyl-5,5,8-trioxo-5lambda*6*-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50070286 (4-Benzenesulfonyl-2-benzyl-3-hydroxy-2H-isoquinoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50056998 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50070288 (2-(3,4-Difluoro-benzyl)-3-hydroxy-4-(propane-2-sul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production; Imax=84% | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50070287 (2-(4-Fluoro-benzyl)-3-hydroxy-4-(propane-2-sulfony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50070290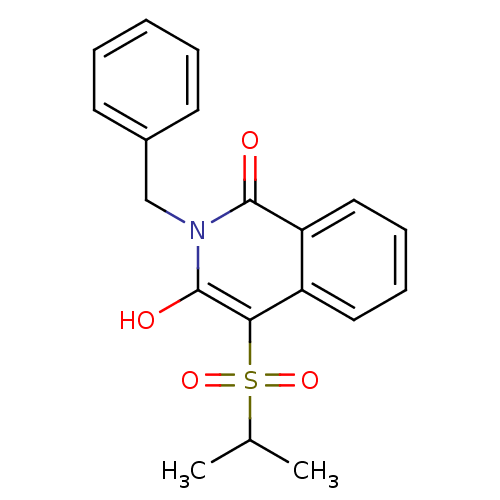 (2-Benzyl-3-hydroxy-4-(propane-2-sulfonyl)-2H-isoqu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50070290 (2-Benzyl-3-hydroxy-4-(propane-2-sulfonyl)-2H-isoqu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004187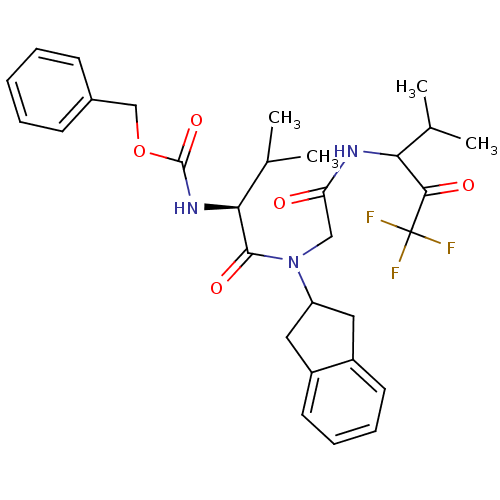 (((S)-1-{Indan-2-yl-[(3,3,3-trifluoro-1-isopropyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 365 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004185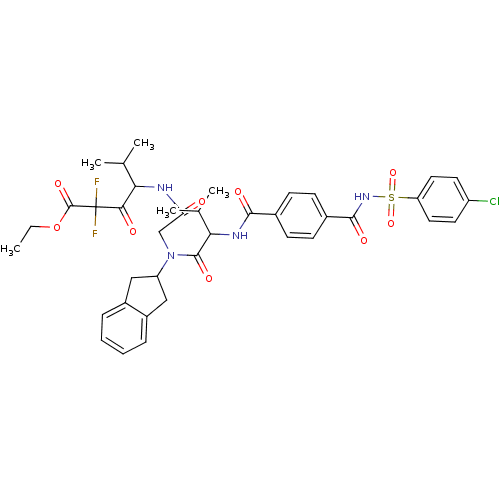 (4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 404 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50070288 (2-(3,4-Difluoro-benzyl)-3-hydroxy-4-(propane-2-sul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004183 (4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 635 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Homo sapiens (Human)) | BDBM50012789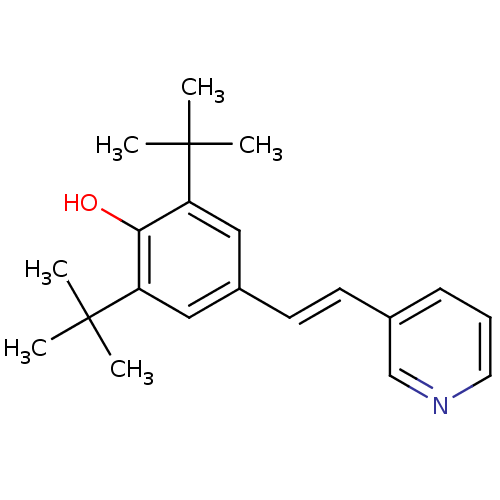 (2,6-Di-tert-butyl-4-(2-pyridin-3-yl-vinyl)-phenol ...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration required for inhibition of Prostaglandin G/H synthase | J Med Chem 32: 100-4 (1989) BindingDB Entry DOI: 10.7270/Q2CN764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50070286 (4-Benzenesulfonyl-2-benzyl-3-hydroxy-2H-isoquinoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50070291 (6-Chloro-2-(3,4-difluoro-benzyl)-3-hydroxy-4-(prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50070289 (2-Benzyl-6-chloro-3-hydroxy-4-(propane-2-sulfonyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50056998 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration that caused a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production. | Bioorg Med Chem Lett 8: 1181-6 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60PJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004189 (4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004188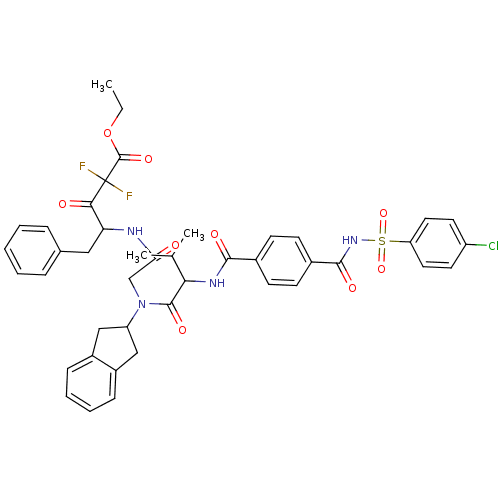 (4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004186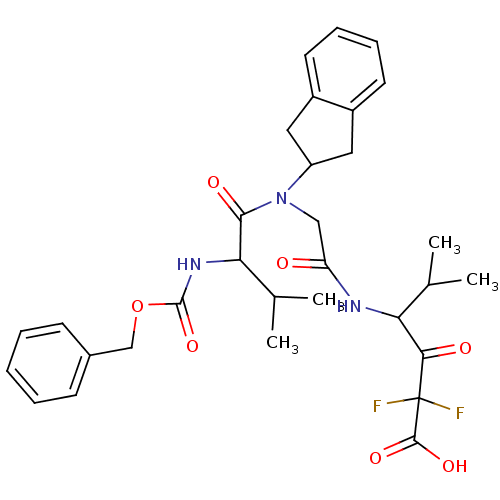 (4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004191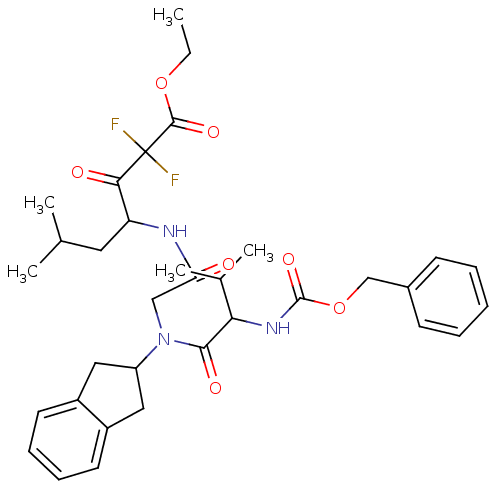 (4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Homo sapiens (Human)) | BDBM22360 (2-(acetyloxy)benzoate | 2-(acetyloxy)benzoic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against cyclooxygenase of human platelets was determined | J Med Chem 30: 726-9 (1987) BindingDB Entry DOI: 10.7270/Q2Q81G93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||