

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132351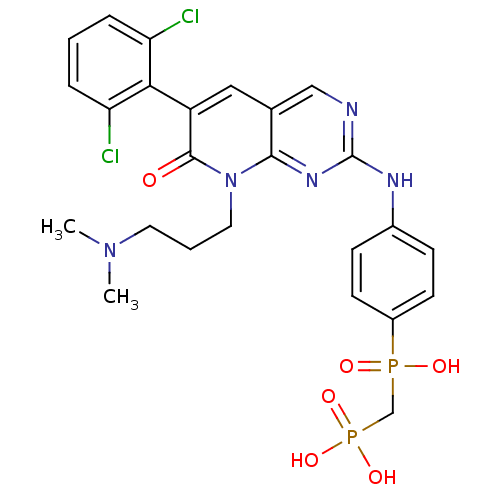 (({4-[6-(2,6-Dichloro-phenyl)-8-(3-dimethylamino-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132348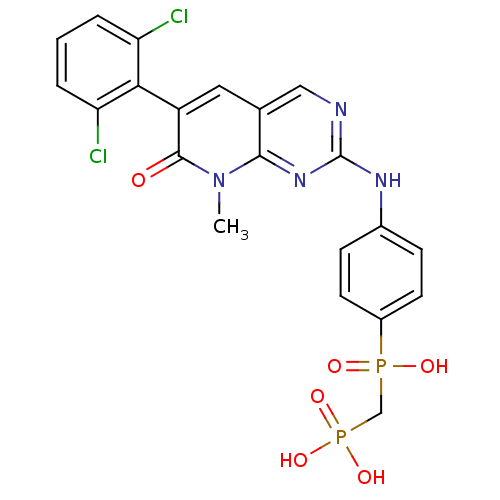 (({4-[6-(2,6-Dichloro-phenyl)-8-methyl-7-oxo-7,8-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM27216 ((2R)-2-({6-[(3-chlorophenyl)amino]-9-(propan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Cyclin-dependent kinase 2 (CDK2) | Bioorg Med Chem Lett 13: 3067-70 (2003) BindingDB Entry DOI: 10.7270/Q25B01V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3071 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one 38 | 2-ani...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50451556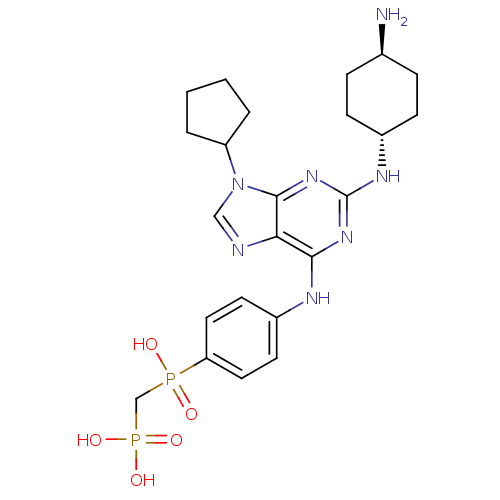 (CHEMBL3084838) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src-mediated dentine resorption in rabbit-osteoclast assay | Bioorg Med Chem Lett 13: 3067-70 (2003) BindingDB Entry DOI: 10.7270/Q25B01V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM50318870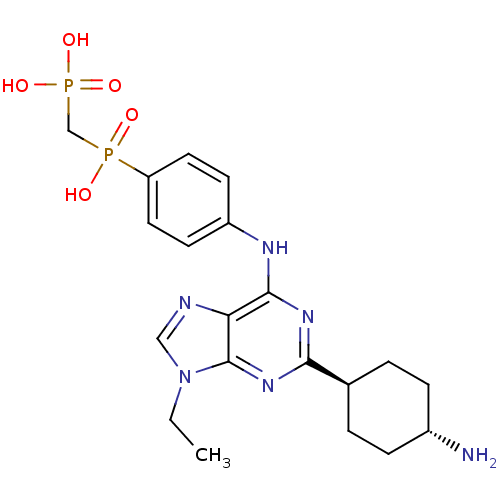 (((4-(2-(cis-4-aminocyclohexyl)-9-ethyl-9H-purin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM81725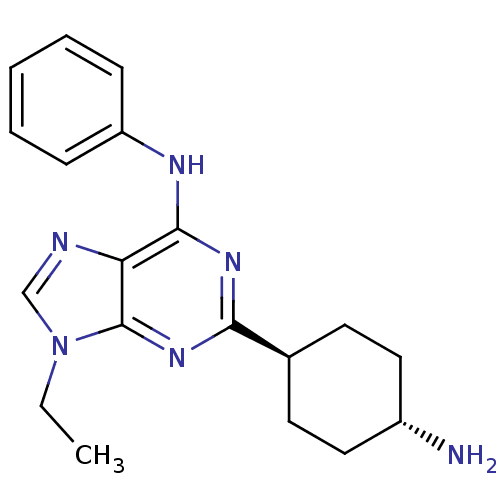 (2,6,9-Trisubstituted Purine, AP23517) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132352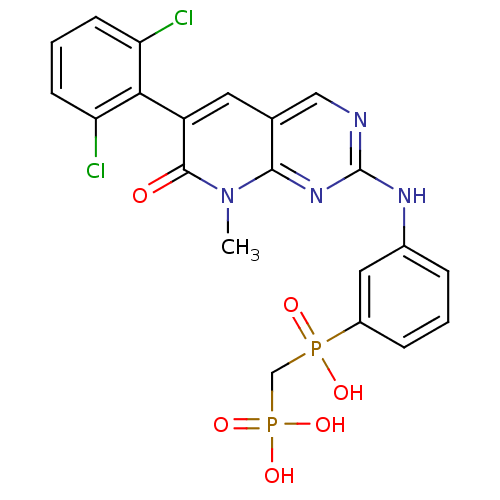 (({3-[6-(2,6-Dichloro-phenyl)-8-methyl-7-oxo-7,8-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM81723 (2,6,9-Trisubstituted Purine, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50451553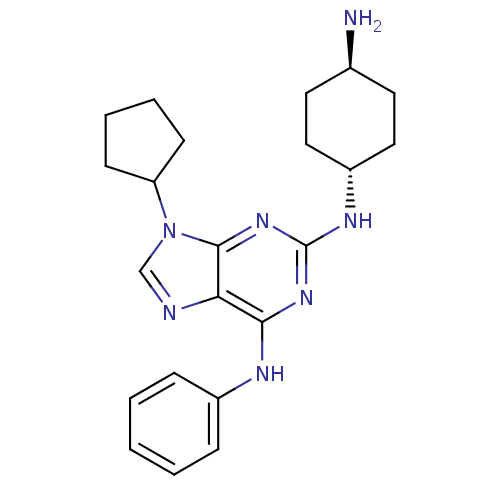 (CHEMBL3084839) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src-mediated dentine resorption in rabbit-osteoclast assay | Bioorg Med Chem Lett 13: 3067-70 (2003) BindingDB Entry DOI: 10.7270/Q25B01V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132353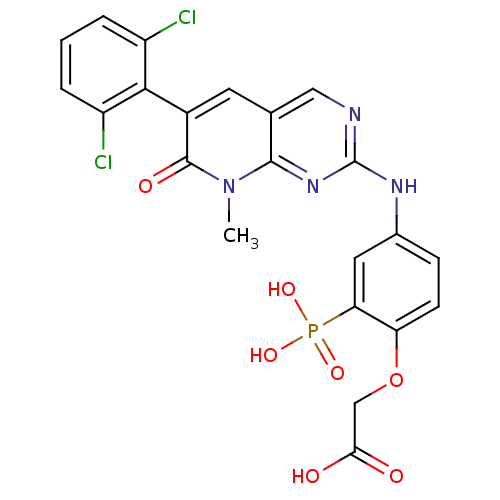 (CHEMBL102801 | {4-[6-(2,6-Dichloro-phenyl)-8-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM81722 (2,6,9-Trisubstituted Purine, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM50318870 (((4-(2-(cis-4-aminocyclohexyl)-9-ethyl-9H-purin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM81718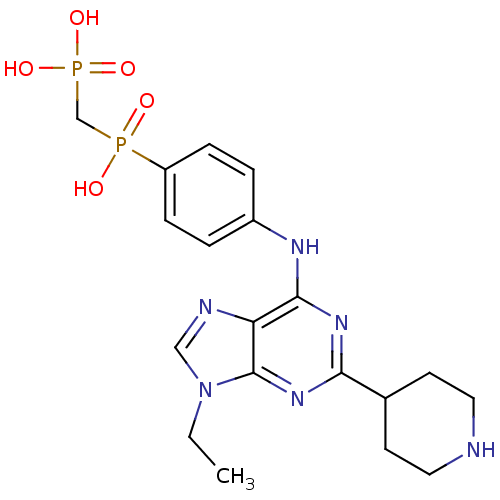 (2,6,9-Trisubstituted Purine, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 665 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132350 (({4-[6-(2,6-Dichloro-phenyl)-8-methyl-7-oxo-7,8-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5106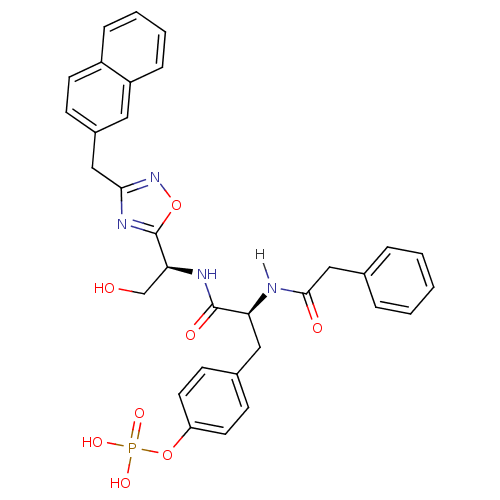 (1,2,4-Oxadiazole Analogue 15c | 4-[(2S)-2-{[(1S)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM81720 (2,6,9-Trisubstituted Purine, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132349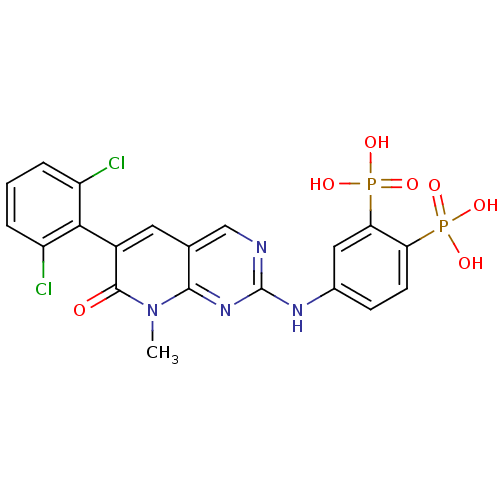 (CHEMBL320374 | {5-[6-(2,6-Dichloro-phenyl)-8-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5105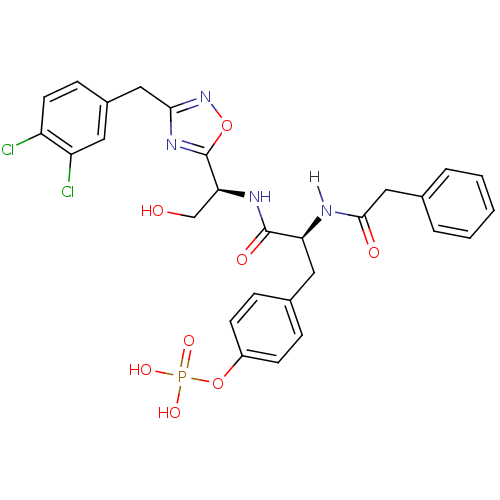 (1,2,4-Oxadiazole Analogue 15b | 4-[(2S)-2-{[(1S)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5103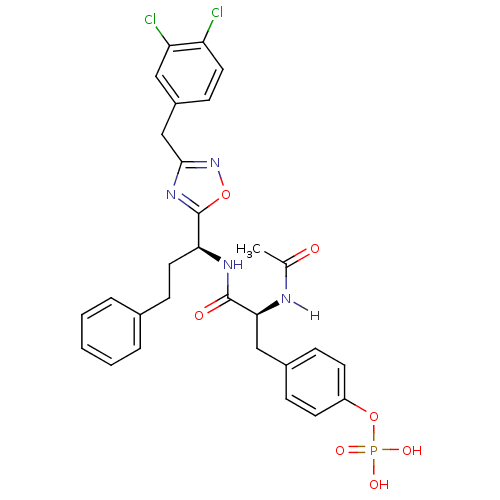 (1,2,4-Oxadiazole Analogue 13c | 4-[(2S)-2-{[(1S)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5099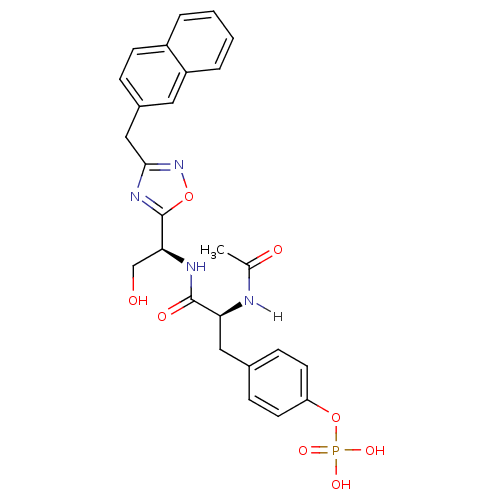 (1,2,4-Oxadiazole Analogue 12c | 4-[(2S)-2-acetamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM81724 (2,6,9-Trisubstituted Purine, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM81721 (2,6,9-Trisubstituted Purine, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM81719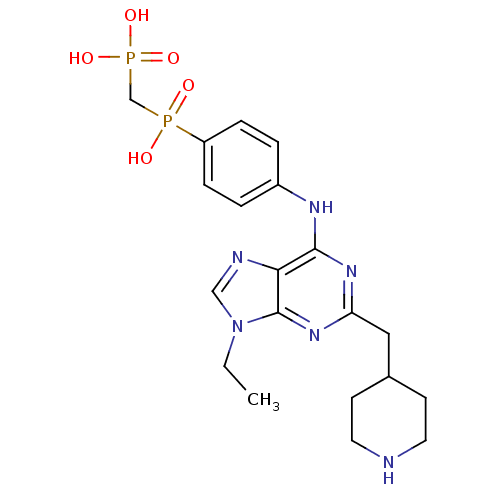 (2,6,9-Trisubstituted Purine, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5104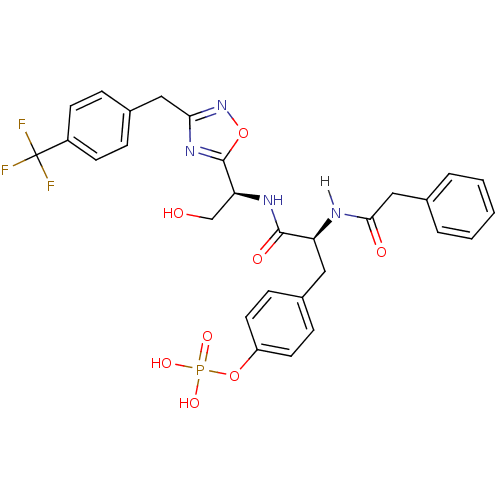 (1,2,4-Oxadiazole Analogue 15a | 4-[(2S)-2-{[(1S)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5102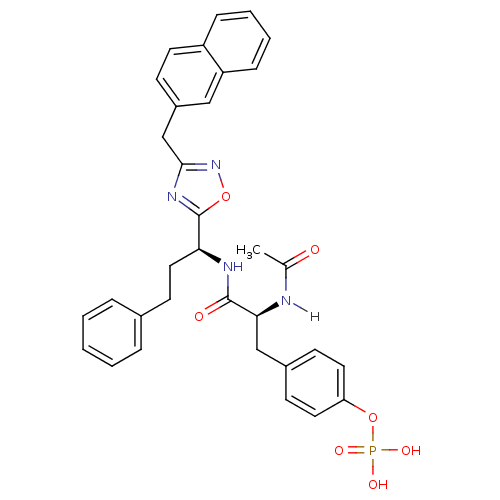 (1,2,4-Oxadiazole Analogue 13b | 4-[(2S)-2-acetamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5090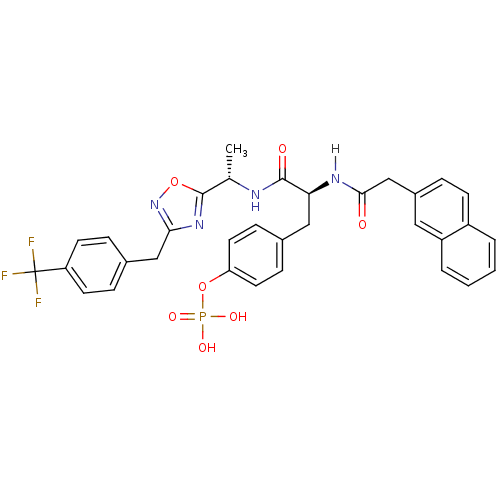 (1,2,4-Oxadiazole Analogue 14e | 4-[(2S)-2-[1-(naph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5086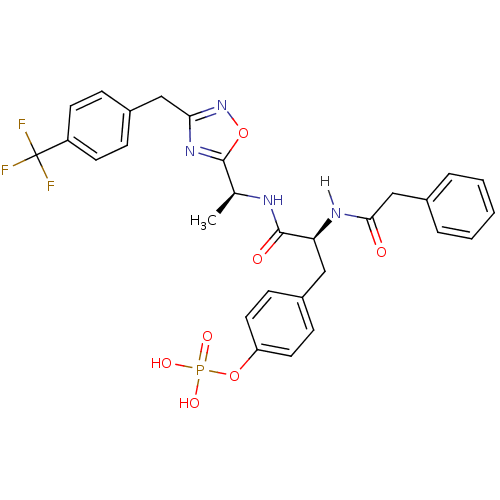 (1,2,4-Oxadiazole Analogue 14a | 4-[(2S)-2-(1-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5074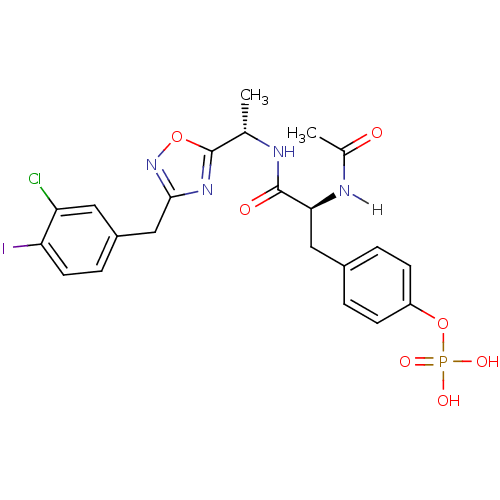 (1,2,4-Oxadiazole Analogue 5m | 4-[(2S)-2-{[(1S)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5075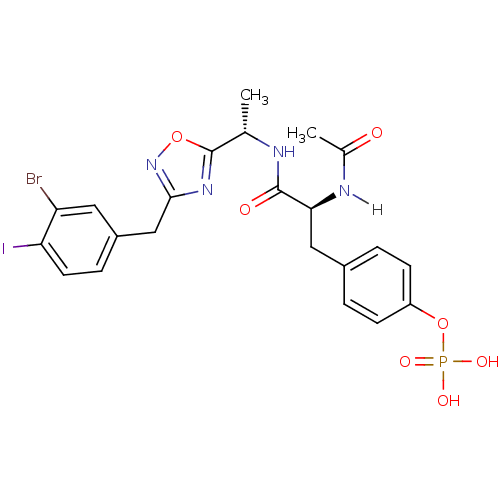 (1,2,4-Oxadiazole Analogue 5n | 4-[(2S)-2-{[(1S)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5098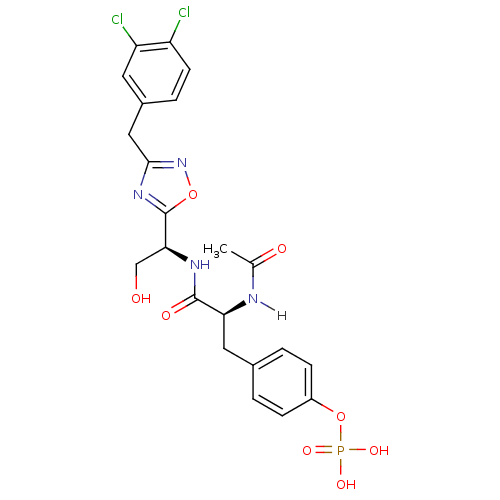 (1,2,4-Oxadiazole Analogue 12b | 4-[(2S)-2-{[(1S)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for Zeta-chain (TCR) associated protein kinase 70 kDa (ZAP70) | Bioorg Med Chem Lett 9: 3009-14 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5098 (1,2,4-Oxadiazole Analogue 12b | 4-[(2S)-2-{[(1S)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5085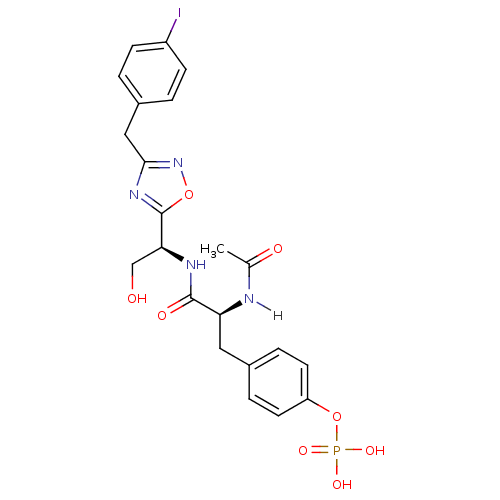 (1,2,4-Oxadiazole Analogue 12g | 4-[(2S)-2-acetamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5072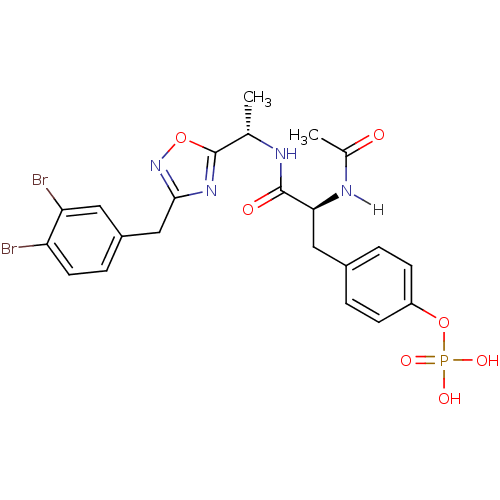 (1,2,4-Oxadiazole Analogue 5k | 4-[(2S)-2-{[(1S)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5100 (1,2,4-Oxadiazole Analogue 12d | 4-[(2S)-2-acetamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5071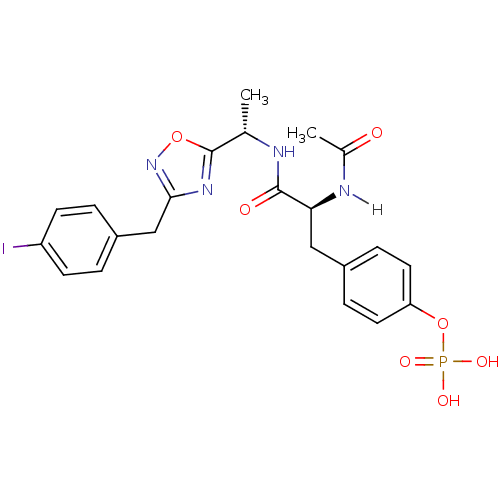 (1,2,4-Oxadiazole Analogue 5j | 4-[(2S)-2-acetamido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5091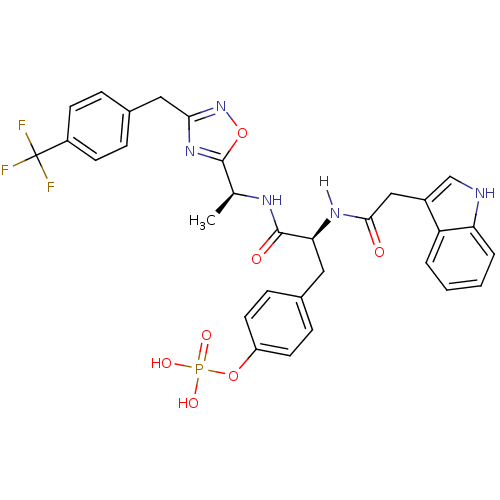 (1,2,4-Oxadiazole Analogue 14f | 4-[(2S)-2-[1-(1H-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5096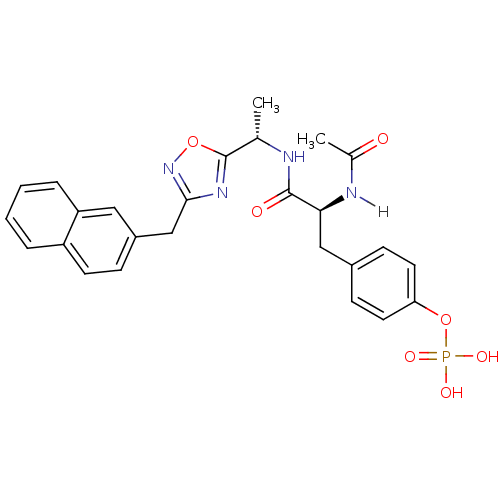 (1,2,4-Oxadiazole Analogue 5e | 4-[(2S)-2-acetamido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5073 (1,2,4-Oxadiazole Analogue 5l | 4-[(2S)-2-{[(1S)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5101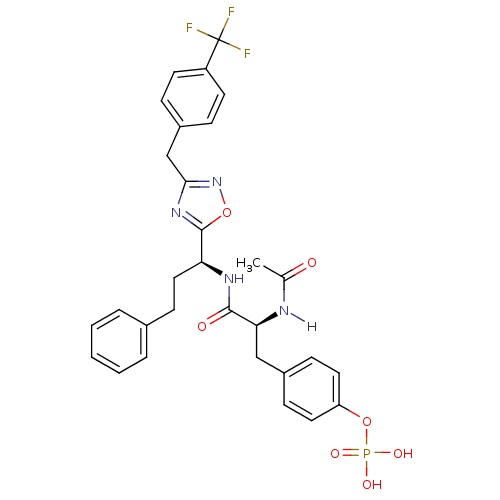 (1,2,4-Oxadiazole Analogue 13a | 4-[(2S)-2-acetamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5087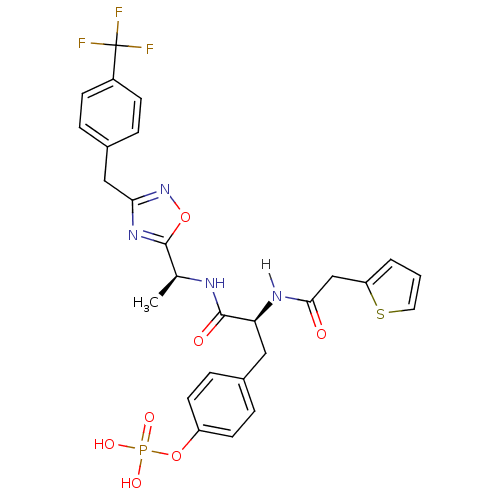 (1,2,4-Oxadiazole Analogue 14b | 4-[(2S)-2-[1-(thio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50451559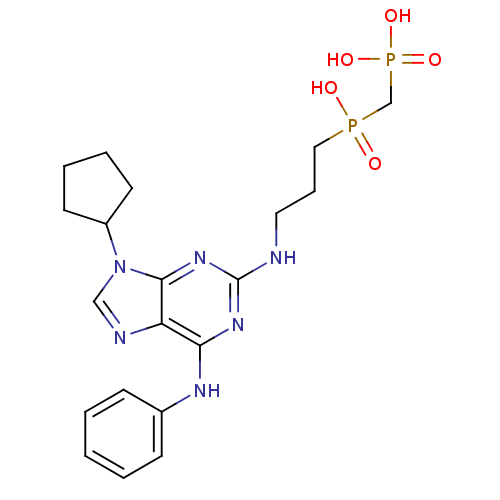 (CHEMBL102550) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src-mediated dentine resorption in rabbit-osteoclast assay | Bioorg Med Chem Lett 13: 3067-70 (2003) BindingDB Entry DOI: 10.7270/Q25B01V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5097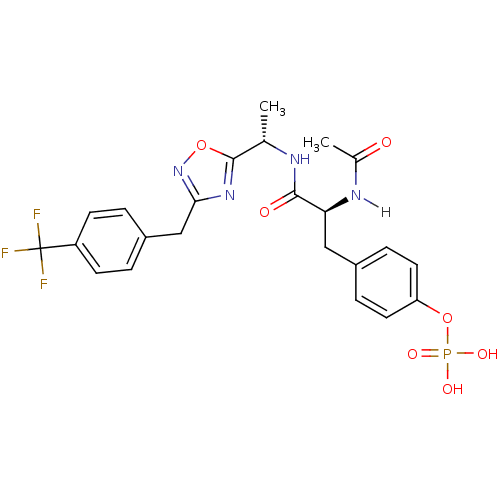 (1,2,4-Oxadiazole Analogue 5f | 4-[(2S)-2-acetamido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for Zeta-chain (TCR) associated protein kinase 70 kDa (ZAP70) | Bioorg Med Chem Lett 9: 3009-14 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5088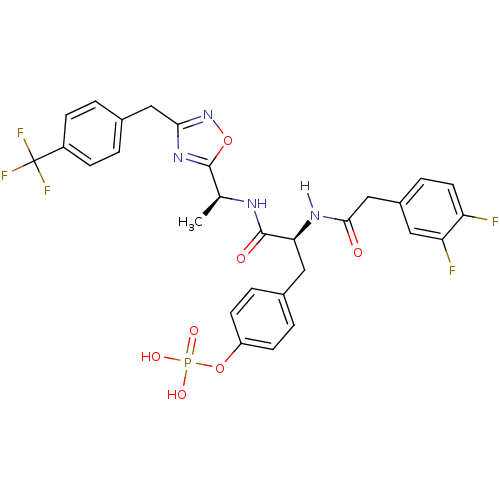 (1,2,4-Oxadiazole Analogue 14c | 4-[(2S)-2-[1-(3,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5089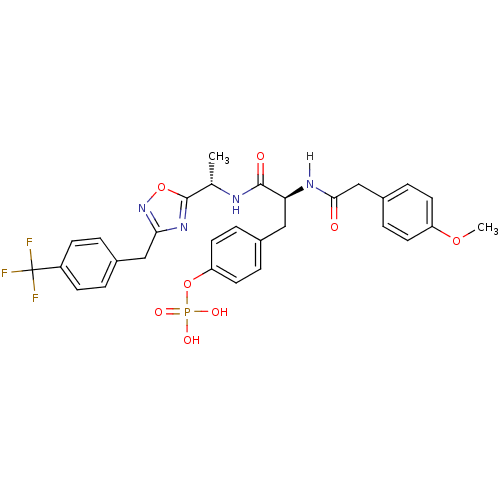 (1,2,4-Oxadiazole Analogue 14d | 4-[(2S)-2-[1-(4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50080177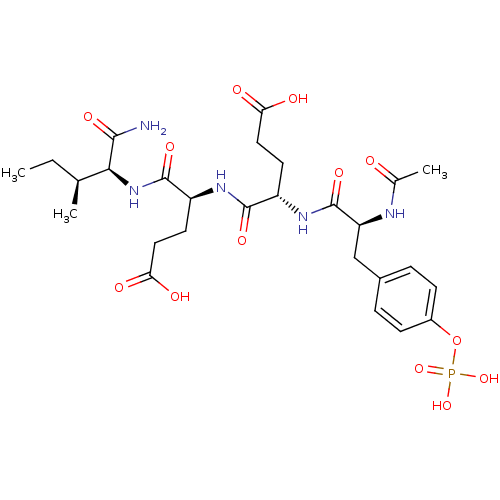 ((S)-4-{(S)-2-[(S)-2-Acetylamino-3-(4-phosphonooxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against Src-homology 2 (SRC SH2). | Bioorg Med Chem Lett 9: 2359-64 (1999) BindingDB Entry DOI: 10.7270/Q2G73CX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5095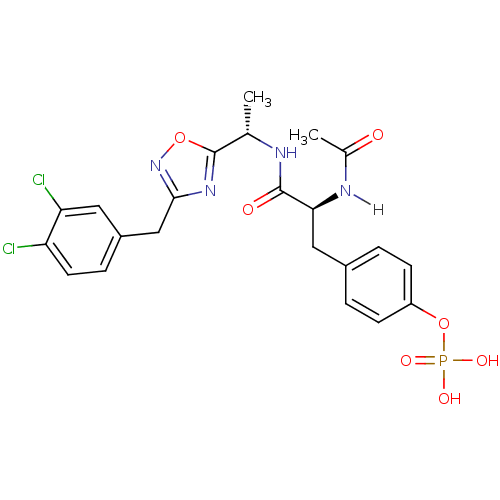 (1,2,4-Oxadiazole Analogue 5d | 4-[(2S)-2-{[(1S)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM5095 (1,2,4-Oxadiazole Analogue 5d | 4-[(2S)-2-{[(1S)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for Zeta-chain (TCR) associated protein kinase 70 kDa (ZAP70) | Bioorg Med Chem Lett 9: 3009-14 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM50082328 (CHEMBL102766 | N-{1-[3-(3,4-Dichloro-benzyl)-[1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Affinity for Zeta-chain (TCR) associated protein kinase 70 kDa (ZAP70) | Bioorg Med Chem Lett 9: 3009-14 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2VQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50080198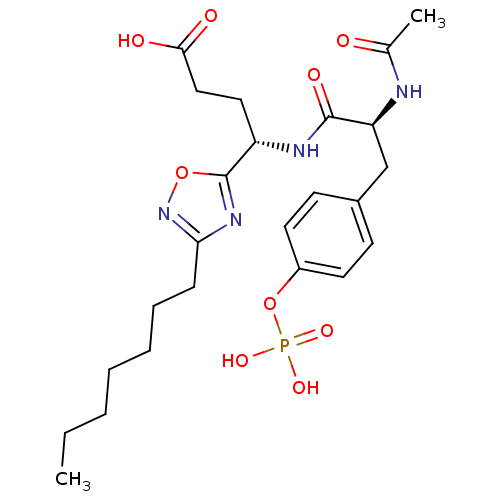 ((S)-4-[(S)-2-Acetylamino-3-(4-phosphonooxy-phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against Src-homology 2 (SRC SH2). | Bioorg Med Chem Lett 9: 2359-64 (1999) BindingDB Entry DOI: 10.7270/Q2G73CX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 235 total ) | Next | Last >> |