

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50281126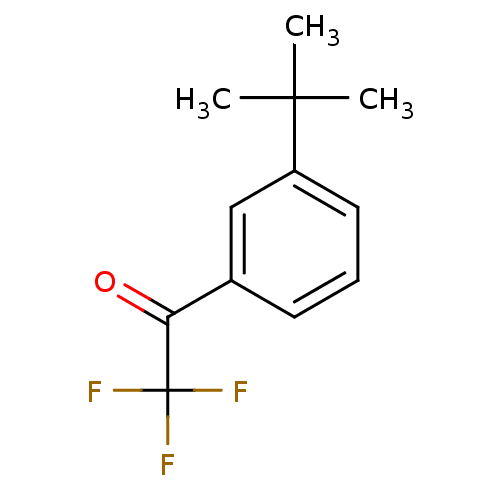 (1-(3-tert-Butyl-phenyl)-2,2,2-trifluoro-ethanone |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.00370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to inhibit Acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 3: 2619-2622 (1993) Article DOI: 10.1016/S0960-894X(01)80727-7 BindingDB Entry DOI: 10.7270/Q2VX0GF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537072 (CHEMBL440072) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50281127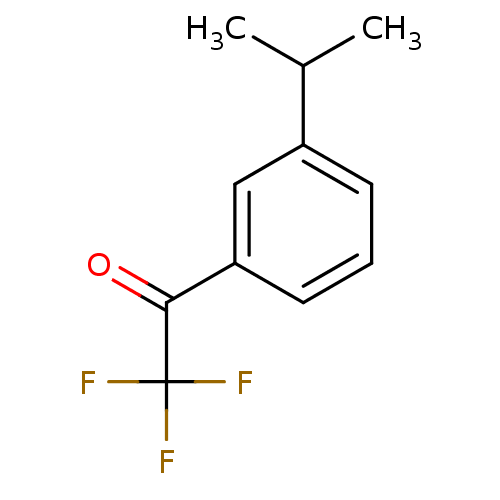 (2,2,2-Trifluoro-1-(3-isopropyl-phenyl)-ethanone | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | 0.00850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to inhibit Acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 3: 2619-2622 (1993) Article DOI: 10.1016/S0960-894X(01)80727-7 BindingDB Entry DOI: 10.7270/Q2VX0GF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537063 (CHEMBL4590517) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537069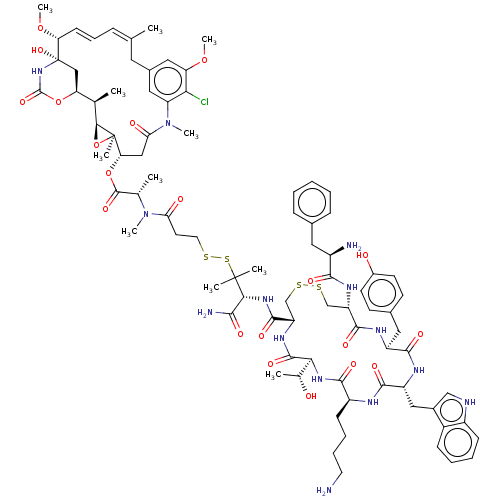 (CHEMBL4584764) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537066 (CHEMBL4541310) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537077 (CHEMBL4550617) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537076 (CHEMBL4564727) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537061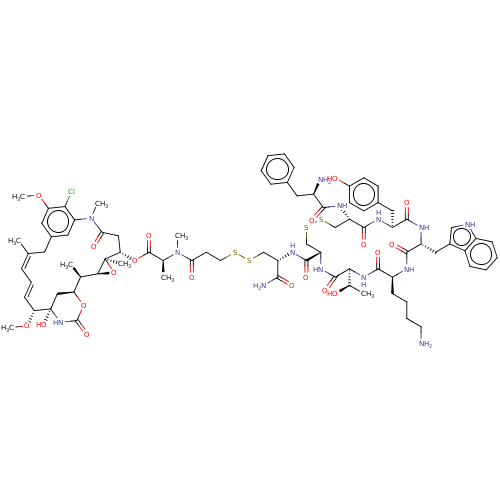 (CHEMBL4527856) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458771 (CHEMBL4214046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537068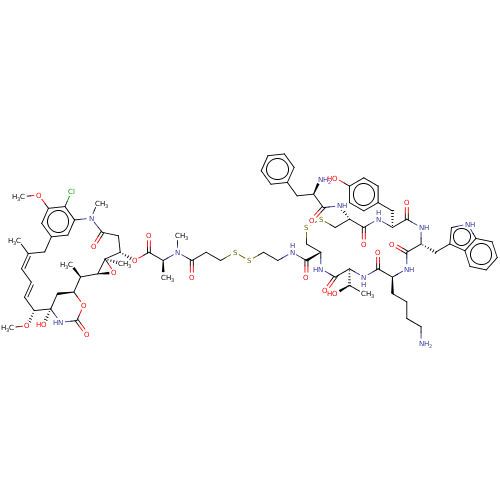 (CHEMBL4592483) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537070 (CHEMBL4581874) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537074 (CHEMBL4556000) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537067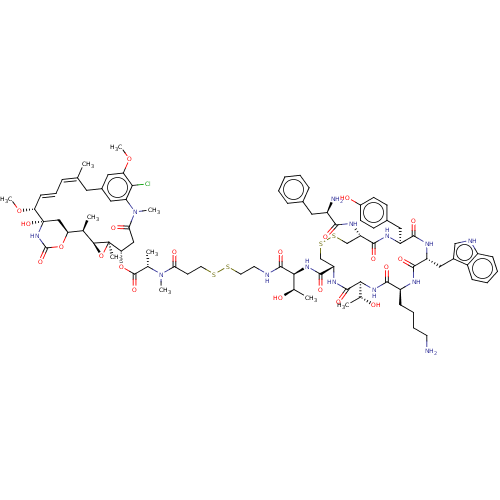 (CHEMBL4532058) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537062 (CHEMBL4549303) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537064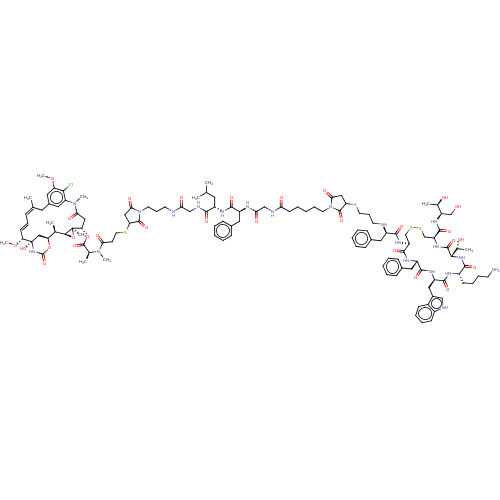 (CHEMBL4563111) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537075 (CHEMBL4548228) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537071 (CHEMBL4581646) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537065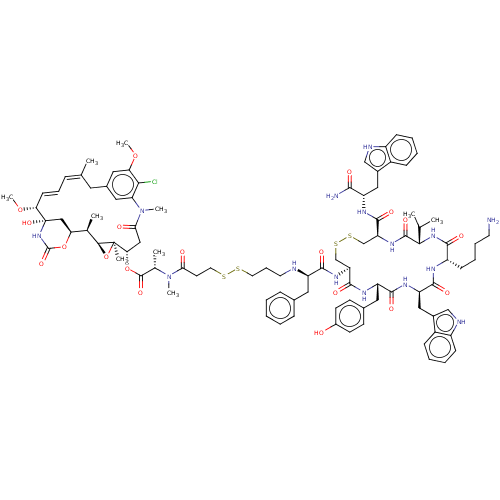 (CHEMBL4537192) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50565846 (CHEMBL4796315) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I] [NIe75, Tyr77]Pyr-apelin-13 from YFP-tagged human APJ receptor expressed in HEK293 cell membranes incubated for 1 hr by gamma ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01547 BindingDB Entry DOI: 10.7270/Q2V40ZZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537078 (CHEMBL4577466) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50281123 (2,2,2-Trifluoro-1-(3-trifluoromethyl-phenyl)-ethan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to inhibit Acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 3: 2619-2622 (1993) Article DOI: 10.1016/S0960-894X(01)80727-7 BindingDB Entry DOI: 10.7270/Q2VX0GF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50281124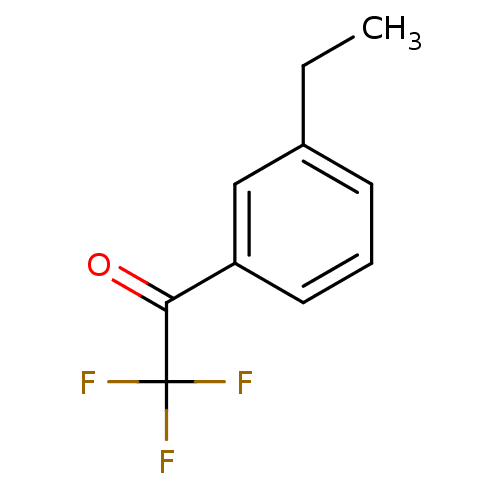 (1-(3-Ethyl-phenyl)-2,2,2-trifluoro-ethanone | CHEM...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to inhibit Acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 3: 2619-2622 (1993) Article DOI: 10.1016/S0960-894X(01)80727-7 BindingDB Entry DOI: 10.7270/Q2VX0GF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50014619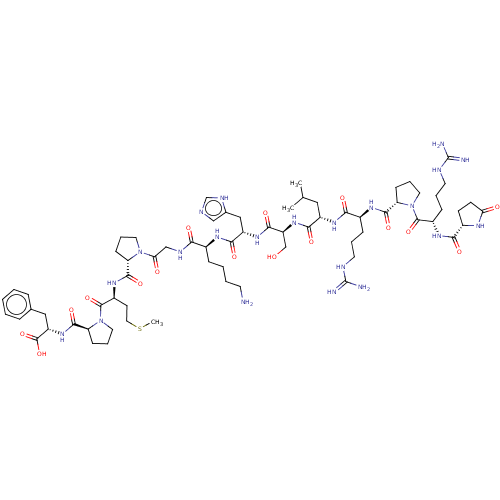 (CHEMBL3184840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I] [NIe75, Tyr77]Pyr-apelin-13 from YFP-tagged human APJ receptor expressed in HEK293 cell membranes incubated for 1 hr by gamma ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01547 BindingDB Entry DOI: 10.7270/Q2V40ZZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537073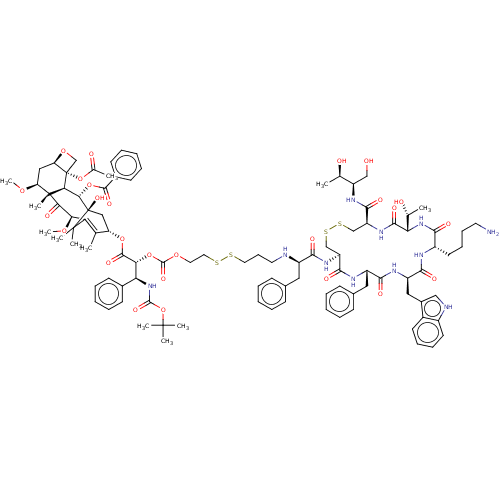 (CHEMBL4534477) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50007887 (3-Methyl-2-oxo-2,3-dihydro-benzoimidazole-1-carbox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto De Angeli Curated by ChEMBL | Assay Description Concentration that inhibits the binding of radioligand, [3H]-ICS 205930, to 5-hydroxytryptamine 3 receptor from rat cortex | J Med Chem 33: 2101-8 (1990) BindingDB Entry DOI: 10.7270/Q22Z164J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50565847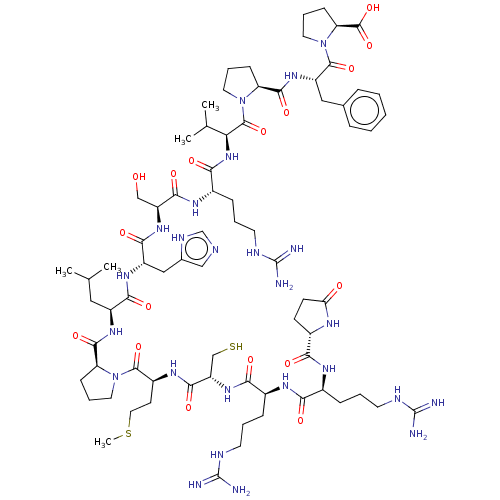 (CHEMBL4780368) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE KEGG PC cid PC sid UniChem | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I] [NIe75, Tyr77]Pyr-apelin-13 from YFP-tagged human APJ receptor expressed in HEK293 cell membranes incubated for 1 hr by gamma ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01547 BindingDB Entry DOI: 10.7270/Q2V40ZZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537060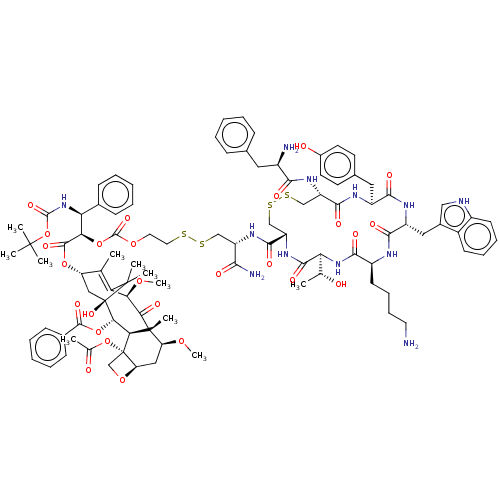 (CHEMBL4575530) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50565907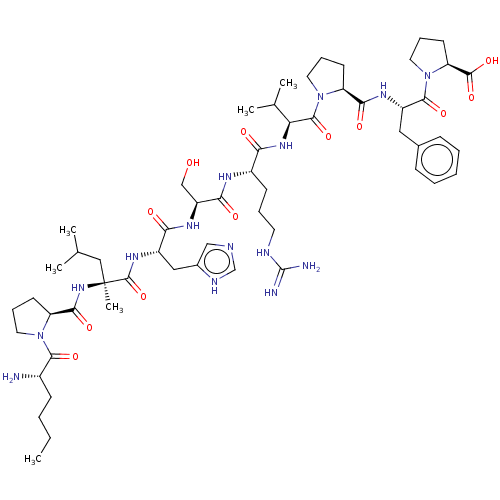 (CHEMBL4791583) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I] [NIe75, Tyr77]Pyr-apelin-13 from YFP-tagged human APJ receptor expressed in HEK293 cell membranes incubated for 1 hr by gamma ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01547 BindingDB Entry DOI: 10.7270/Q2V40ZZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50007868 (2-Oxo-2,3-dihydro-benzoimidazole-1-carboxylic acid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto De Angeli Curated by ChEMBL | Assay Description Concentration that inhibits the binding of radioligand, [3H]-ICS 205930, to 5-hydroxytryptamine 3 receptor from rat cortex | J Med Chem 33: 2101-8 (1990) BindingDB Entry DOI: 10.7270/Q22Z164J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50007879 (3-Methyl-2-oxo-2,3-dihydro-benzoimidazole-1-carbox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto De Angeli Curated by ChEMBL | Assay Description Concentration that inhibits the binding of radioligand, [3H]-ICS 205930, to 5-hydroxytryptamine 3 receptor from rat cortex | J Med Chem 33: 2101-8 (1990) BindingDB Entry DOI: 10.7270/Q22Z164J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto De Angeli Curated by ChEMBL | Assay Description Concentration that inhibits the binding of radioligand, [3H]-ICS 205930, to 5-hydroxytryptamine 3 receptor from rat cortex | J Med Chem 33: 2101-8 (1990) BindingDB Entry DOI: 10.7270/Q22Z164J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50007884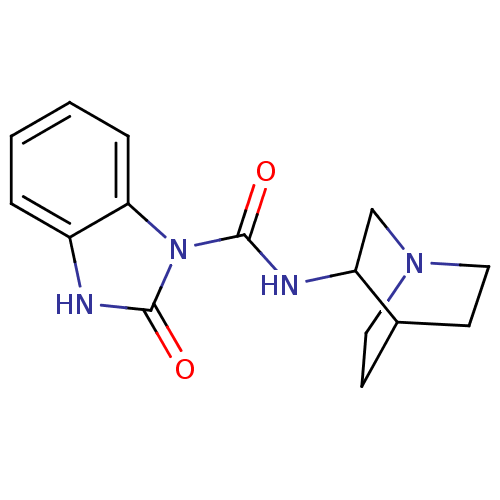 (2-Oxo-2,3-dihydro-benzoimidazole-1-carboxylic acid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto De Angeli Curated by ChEMBL | Assay Description Concentration that inhibits the binding of radioligand, [3H]-ICS 205930, to 5-hydroxytryptamine 3 receptor from rat cortex | J Med Chem 33: 2101-8 (1990) BindingDB Entry DOI: 10.7270/Q22Z164J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50007870 (2-Oxo-2,3-dihydro-benzoimidazole-1-carboxylic acid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto De Angeli Curated by ChEMBL | Assay Description Concentration that inhibits the binding of radioligand, [3H]-ICS 205930, to 5-hydroxytryptamine 3 receptor from rat cortex | J Med Chem 33: 2101-8 (1990) BindingDB Entry DOI: 10.7270/Q22Z164J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50007872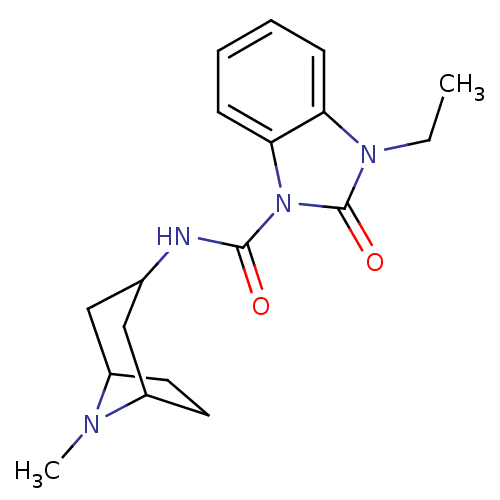 (3-Ethyl-2-oxo-2,3-dihydro-benzoimidazole-1-carboxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto De Angeli Curated by ChEMBL | Assay Description Concentration that inhibits the binding of radioligand, [3H]-ICS 205930, to 5-hydroxytryptamine 3 receptor from rat cortex | J Med Chem 33: 2101-8 (1990) BindingDB Entry DOI: 10.7270/Q22Z164J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50565914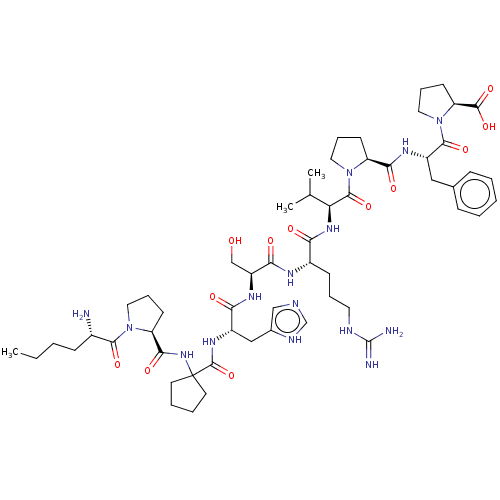 (CHEMBL4797921) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I] [NIe75, Tyr77]Pyr-apelin-13 from YFP-tagged human APJ receptor expressed in HEK293 cell membranes incubated for 1 hr by gamma ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01547 BindingDB Entry DOI: 10.7270/Q2V40ZZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50281125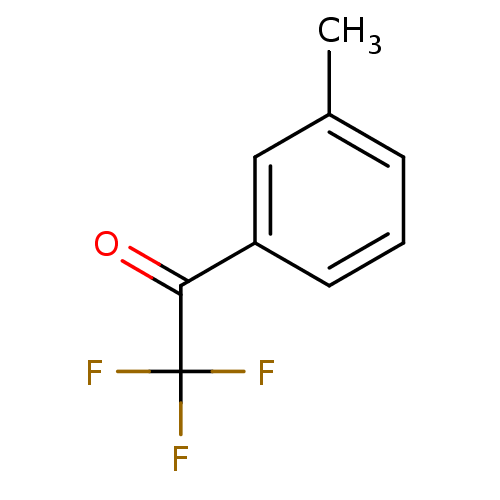 (2,2,2-Trifluoro-1-m-tolyl-ethanone | CHEMBL86868) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for the ability to inhibit Acetylcholinesterase (AChE) from Torpedo californica | Bioorg Med Chem Lett 3: 2619-2622 (1993) Article DOI: 10.1016/S0960-894X(01)80727-7 BindingDB Entry DOI: 10.7270/Q2VX0GF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50007886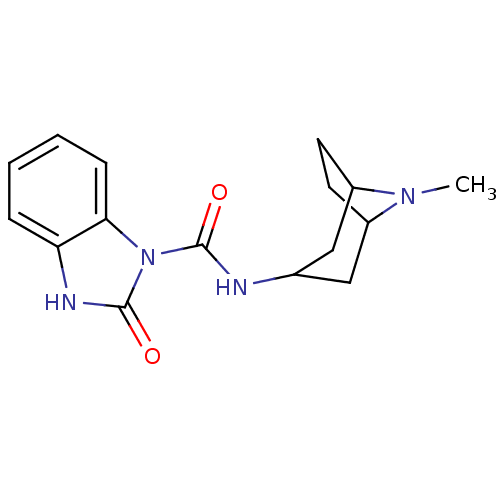 (2-Oxo-2,3-dihydro-benzoimidazole-1-carboxylic acid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto De Angeli Curated by ChEMBL | Assay Description Concentration that inhibits the binding of radioligand, [3H]-ICS 205930, to 5-hydroxytryptamine 3 receptor from rat cortex | J Med Chem 33: 2101-8 (1990) BindingDB Entry DOI: 10.7270/Q22Z164J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50565876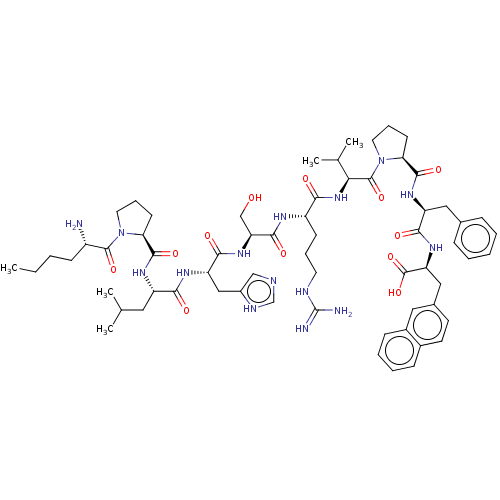 (CHEMBL4781348) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I] [NIe75, Tyr77]Pyr-apelin-13 from YFP-tagged human APJ receptor expressed in HEK293 cell membranes incubated for 1 hr by gamma ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01547 BindingDB Entry DOI: 10.7270/Q2V40ZZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50565911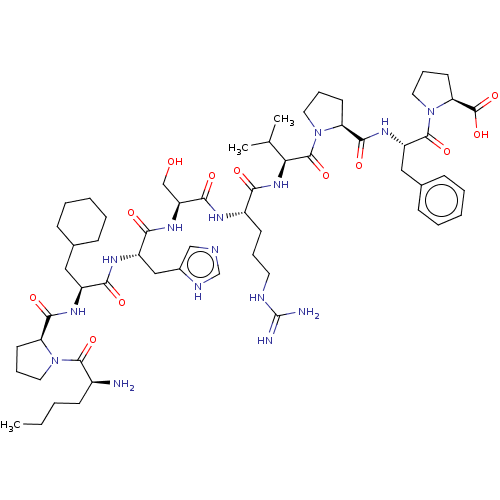 (CHEMBL4790636) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I] [NIe75, Tyr77]Pyr-apelin-13 from YFP-tagged human APJ receptor expressed in HEK293 cell membranes incubated for 1 hr by gamma ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01547 BindingDB Entry DOI: 10.7270/Q2V40ZZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50565873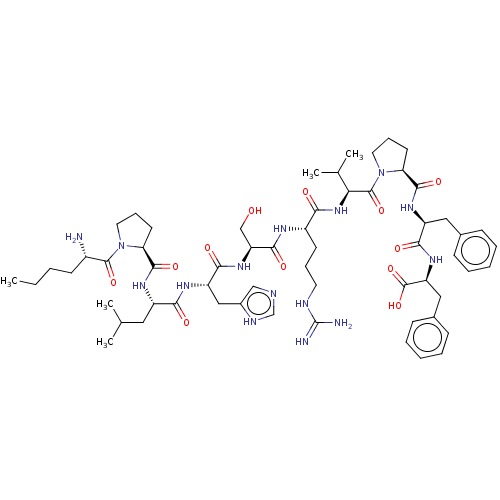 (CHEMBL4777690) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I] [NIe75, Tyr77]Pyr-apelin-13 from YFP-tagged human APJ receptor expressed in HEK293 cell membranes incubated for 1 hr by gamma ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01547 BindingDB Entry DOI: 10.7270/Q2V40ZZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50565880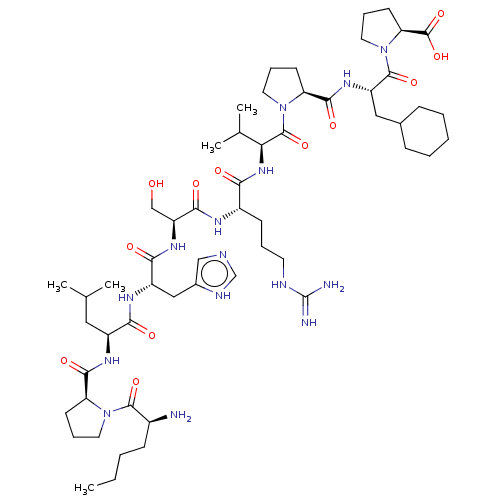 (CHEMBL4794333) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I] [NIe75, Tyr77]Pyr-apelin-13 from YFP-tagged human APJ receptor expressed in HEK293 cell membranes incubated for 1 hr by gamma ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01547 BindingDB Entry DOI: 10.7270/Q2V40ZZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50565853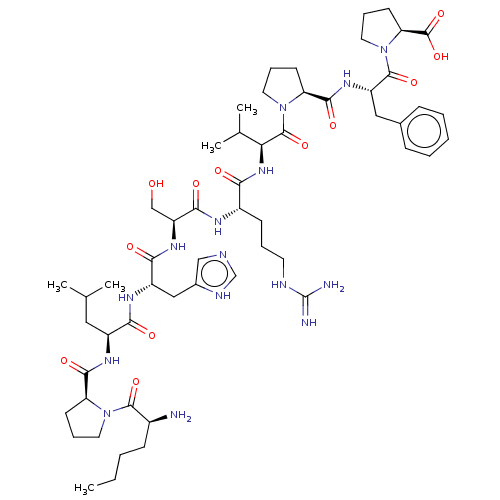 (CHEMBL4788436) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I] [NIe75, Tyr77]Pyr-apelin-13 from YFP-tagged human APJ receptor expressed in HEK293 cell membranes incubated for 1 hr by gamma ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01547 BindingDB Entry DOI: 10.7270/Q2V40ZZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50565878 (CHEMBL4797169) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I] [NIe75, Tyr77]Pyr-apelin-13 from YFP-tagged human APJ receptor expressed in HEK293 cell membranes incubated for 1 hr by gamma ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01547 BindingDB Entry DOI: 10.7270/Q2V40ZZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50586618 (CHEMBL5078775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human neutrophil elastase | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128380 BindingDB Entry DOI: 10.7270/Q28919SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1976 total ) | Next | Last >> |