 Found 188 hits with Last Name = 'raidaru' and Initial = 'g'
Found 188 hits with Last Name = 'raidaru' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50311411
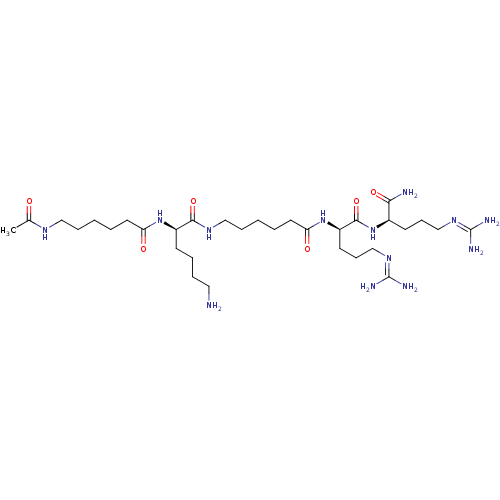
((R)-2-(6-acetamidohexanamido)-6-amino-N-(6-((R)-1-...)Show SMILES [#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C32H63N13O6/c1-22(46)39-18-8-2-4-15-26(47)43-24(12-6-7-17-33)29(50)40-19-9-3-5-16-27(48)44-25(14-11-21-42-32(37)38)30(51)45-23(28(34)49)13-10-20-41-31(35)36/h23-25H,2-21,33H2,1H3,(H2,34,49)(H,39,46)(H,40,50)(H,43,47)(H,44,48)(H,45,51)(H4,35,36,41)(H4,37,38,42)/t23-,24-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of PKBgamma in presence of 100 uM ATP |
Bioorg Med Chem Lett 19: 6098-101 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.026
BindingDB Entry DOI: 10.7270/Q2JM29R4 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Mus musculus (mouse)) | BDBM27250
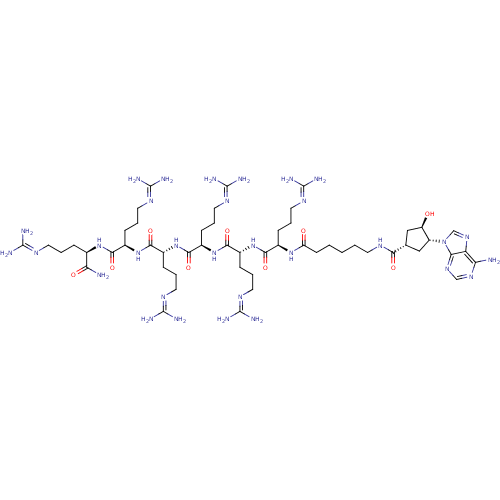
(6-{[(1S,3R,4R)-3-(6-amino-9H-purin-9-yl)-4-hydroxy...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6@@H](-[#8])-[#6@@H](-[#6]-1)-n1cnc2c(-[#7])ncnc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C53H97N31O9/c54-39-38-41(76-26-75-39)84(27-77-38)35-24-28(25-36(35)85)42(88)68-17-3-1-2-16-37(86)78-30(11-5-19-70-49(58)59)43(89)80-32(13-7-21-72-51(62)63)45(91)82-34(15-9-23-74-53(66)67)47(93)83-33(14-8-22-73-52(64)65)46(92)81-31(12-6-20-71-50(60)61)44(90)79-29(40(55)87)10-4-18-69-48(56)57/h26-36,85H,1-25H2,(H2,55,87)(H,68,88)(H,78,86)(H,79,90)(H,80,89)(H,81,92)(H,82,91)(H,83,93)(H2,54,75,76)(H4,56,57,69)(H4,58,59,70)(H4,60,61,71)(H4,62,63,72)(H4,64,65,73)(H4,66,67,74)/t28-,29+,30+,31+,32+,33+,34+,35+,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM27249
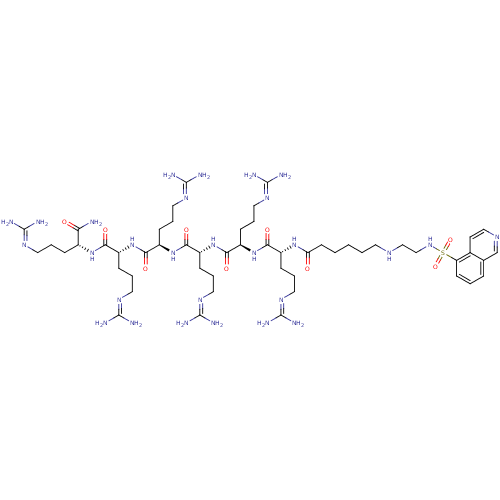
(ARC-903 | N-[(1R)-4-carbamimidamido-1-{[(1R)-4-car...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C53H96N28O9S/c54-42(83)34(12-5-22-69-48(55)56)77-44(85)36(14-7-24-71-50(59)60)79-46(87)38(16-9-26-73-52(63)64)81-47(88)39(17-10-27-74-53(65)66)80-45(86)37(15-8-25-72-51(61)62)78-43(84)35(13-6-23-70-49(57)58)76-41(82)19-2-1-3-21-67-29-30-75-91(89,90)40-18-4-11-32-31-68-28-20-33(32)40/h4,11,18,20,28,31,34-39,67,75H,1-3,5-10,12-17,19,21-27,29-30H2,(H2,54,83)(H,76,82)(H,77,85)(H,78,84)(H,79,87)(H,80,86)(H,81,88)(H4,55,56,69)(H4,57,58,70)(H4,59,60,71)(H4,61,62,72)(H4,63,64,73)(H4,65,66,74)/t34-,35-,36-,37-,38-,39-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-1042 from His6-tagged recombinant human ROCK2 by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM27249

(ARC-903 | N-[(1R)-4-carbamimidamido-1-{[(1R)-4-car...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C53H96N28O9S/c54-42(83)34(12-5-22-69-48(55)56)77-44(85)36(14-7-24-71-50(59)60)79-46(87)38(16-9-26-73-52(63)64)81-47(88)39(17-10-27-74-53(65)66)80-45(86)37(15-8-25-72-51(61)62)78-43(84)35(13-6-23-70-49(57)58)76-41(82)19-2-1-3-21-67-29-30-75-91(89,90)40-18-4-11-32-31-68-28-20-33(32)40/h4,11,18,20,28,31,34-39,67,75H,1-3,5-10,12-17,19,21-27,29-30H2,(H2,54,83)(H,76,82)(H,77,85)(H,78,84)(H,79,87)(H,80,86)(H,81,88)(H4,55,56,69)(H4,57,58,70)(H4,59,60,71)(H4,61,62,72)(H4,63,64,73)(H4,65,66,74)/t34-,35-,36-,37-,38-,39-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-583/ARC-1042/ARC-1059 from His6-tagged recombinant human ROCK2 by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM27249

(ARC-903 | N-[(1R)-4-carbamimidamido-1-{[(1R)-4-car...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C53H96N28O9S/c54-42(83)34(12-5-22-69-48(55)56)77-44(85)36(14-7-24-71-50(59)60)79-46(87)38(16-9-26-73-52(63)64)81-47(88)39(17-10-27-74-53(65)66)80-45(86)37(15-8-25-72-51(61)62)78-43(84)35(13-6-23-70-49(57)58)76-41(82)19-2-1-3-21-67-29-30-75-91(89,90)40-18-4-11-32-31-68-28-20-33(32)40/h4,11,18,20,28,31,34-39,67,75H,1-3,5-10,12-17,19,21-27,29-30H2,(H2,54,83)(H,76,82)(H,77,85)(H,78,84)(H,79,87)(H,80,86)(H,81,88)(H4,55,56,69)(H4,57,58,70)(H4,59,60,71)(H4,61,62,72)(H4,63,64,73)(H4,65,66,74)/t34-,35-,36-,37-,38-,39-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-583/ARC-1042/ARC-1059 from His6-tagged recombinant human ROCK2 by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50311409
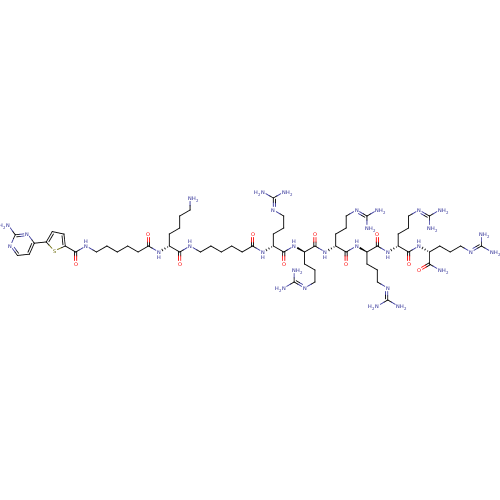
(CHEMBL1077375 | N-((6R,9R,12R,15R,18R,21R,31R)-1-a...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1ccc(s1)-c1ccnc(-[#7])n1)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H114N32O10S/c64-27-6-5-15-39(88-47(96)22-4-2-8-29-80-56(105)46-25-24-45(106-46)37-26-36-87-63(78)95-37)50(99)79-28-7-1-3-23-48(97)89-40(17-10-31-82-58(68)69)51(100)91-42(19-12-33-84-60(72)73)53(102)93-44(21-14-35-86-62(76)77)55(104)94-43(20-13-34-85-61(74)75)54(103)92-41(18-11-32-83-59(70)71)52(101)90-38(49(65)98)16-9-30-81-57(66)67/h24-26,36,38-44H,1-23,27-35,64H2,(H2,65,98)(H,79,99)(H,80,105)(H,88,96)(H,89,97)(H,90,101)(H,91,100)(H,92,103)(H,93,102)(H,94,104)(H4,66,67,81)(H4,68,69,82)(H4,70,71,83)(H4,72,73,84)(H4,74,75,85)(H4,76,77,86)(H2,78,87,95)/t38-,39-,40-,41-,42-,43-,44-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-1063 from His6-tagged recombinant human ROCK2 by luminescence intensity assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50311410
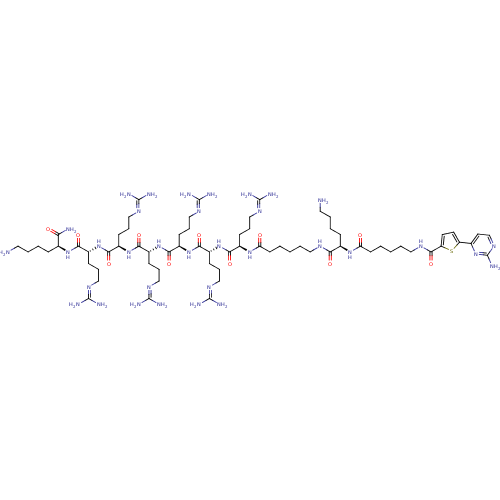
(CHEMBL1077376 | N-((5S,8R,11R,14R,17R,20R,23R,33R)...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1ccc(s1)-c1ccnc(-[#7])n1)-[#6](-[#7])=O |r| Show InChI InChI=1S/C69H126N34O11S/c70-30-7-5-17-42(54(72)106)97-57(109)45(20-12-35-89-64(75)76)99-59(111)47(22-14-37-91-66(79)80)101-61(113)49(24-16-39-93-68(83)84)102-60(112)48(23-15-38-92-67(81)82)100-58(110)46(21-13-36-90-65(77)78)98-56(108)44(19-11-34-88-63(73)74)96-53(105)26-3-1-9-32-86-55(107)43(18-6-8-31-71)95-52(104)25-4-2-10-33-87-62(114)51-28-27-50(115-51)41-29-40-94-69(85)103-41/h27-29,40,42-49H,1-26,30-39,70-71H2,(H2,72,106)(H,86,107)(H,87,114)(H,95,104)(H,96,105)(H,97,109)(H,98,108)(H,99,111)(H,100,110)(H,101,113)(H,102,112)(H4,73,74,88)(H4,75,76,89)(H4,77,78,90)(H4,79,80,91)(H4,81,82,92)(H4,83,84,93)(H2,85,94,103)/t42-,43+,44+,45+,46+,47+,48+,49+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of PKACalpha in presence of 1000 uM ATP |
Bioorg Med Chem Lett 19: 6098-101 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.026
BindingDB Entry DOI: 10.7270/Q2JM29R4 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Mus musculus (mouse)) | BDBM27249

(ARC-903 | N-[(1R)-4-carbamimidamido-1-{[(1R)-4-car...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C53H96N28O9S/c54-42(83)34(12-5-22-69-48(55)56)77-44(85)36(14-7-24-71-50(59)60)79-46(87)38(16-9-26-73-52(63)64)81-47(88)39(17-10-27-74-53(65)66)80-45(86)37(15-8-25-72-51(61)62)78-43(84)35(13-6-23-70-49(57)58)76-41(82)19-2-1-3-21-67-29-30-75-91(89,90)40-18-4-11-32-31-68-28-20-33(32)40/h4,11,18,20,28,31,34-39,67,75H,1-3,5-10,12-17,19,21-27,29-30H2,(H2,54,83)(H,76,82)(H,77,85)(H,78,84)(H,79,87)(H,80,86)(H,81,88)(H4,55,56,69)(H4,57,58,70)(H4,59,60,71)(H4,61,62,72)(H4,63,64,73)(H4,65,66,74)/t34-,35-,36-,37-,38-,39-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50199310

(2S,3S,4R,5R)-5-6-amino-9H-purin-9-yl)-N-12R,15R,18...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)NC(=O)CCCCCNCCNS(=O)(=O)c1cccc2cnccc12 Show InChI InChI=1S/C69H116N34O14S/c70-54-50-55(93-37-92-54)103(38-94-50)63-52(107)51(106)53(117-63)62(114)85-26-6-2-4-22-48(104)96-41(15-8-27-86-64(71)72)56(108)97-42(16-9-28-87-65(73)74)57(109)98-43(17-10-29-88-66(75)76)58(110)99-44(18-11-30-89-67(77)78)59(111)100-45(19-12-31-90-68(79)80)60(112)101-46(20-13-32-91-69(81)82)61(113)102-49(105)23-3-1-5-25-83-34-35-95-118(115,116)47-21-7-14-39-36-84-33-24-40(39)47/h7,14,21,24,33,36-38,41-46,51-53,63,83,95,106-107H,1-6,8-13,15-20,22-23,25-32,34-35H2,(H,85,114)(H,96,104)(H,97,108)(H,98,109)(H,99,110)(H,100,111)(H,101,112)(H2,70,92,93)(H4,71,72,86)(H4,73,74,87)(H4,75,76,88)(H4,77,78,89)(H4,79,80,90)(H4,81,82,91)(H,102,105,113)/t41-,42-,43-,44-,45-,46-,51+,52-,53+,63-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic and Bioorganic Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory potency towards human cAPK C alpha in the presence of 100uM ATP and 30uM TAMRA-kemptide |
J Med Chem 49: 7150-9 (2006)
Article DOI: 10.1021/jm0605942
BindingDB Entry DOI: 10.7270/Q2542N79 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50199310

(2S,3S,4R,5R)-5-6-amino-9H-purin-9-yl)-N-12R,15R,18...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)CCCCCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)NC(=O)CCCCCNCCNS(=O)(=O)c1cccc2cnccc12 Show InChI InChI=1S/C69H116N34O14S/c70-54-50-55(93-37-92-54)103(38-94-50)63-52(107)51(106)53(117-63)62(114)85-26-6-2-4-22-48(104)96-41(15-8-27-86-64(71)72)56(108)97-42(16-9-28-87-65(73)74)57(109)98-43(17-10-29-88-66(75)76)58(110)99-44(18-11-30-89-67(77)78)59(111)100-45(19-12-31-90-68(79)80)60(112)101-46(20-13-32-91-69(81)82)61(113)102-49(105)23-3-1-5-25-83-34-35-95-118(115,116)47-21-7-14-39-36-84-33-24-40(39)47/h7,14,21,24,33,36-38,41-46,51-53,63,83,95,106-107H,1-6,8-13,15-20,22-23,25-32,34-35H2,(H,85,114)(H,96,104)(H,97,108)(H,98,109)(H,99,110)(H,100,111)(H,101,112)(H2,70,92,93)(H4,71,72,86)(H4,73,74,87)(H4,75,76,88)(H4,77,78,89)(H4,79,80,90)(H4,81,82,91)(H,102,105,113)/t41-,42-,43-,44-,45-,46-,51+,52-,53+,63-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic and Bioorganic Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory potency towards human cAPK C alpha in the presence of 100uM ATP and 30uM TAMRA-kemptide |
J Med Chem 49: 7150-9 (2006)
Article DOI: 10.1021/jm0605942
BindingDB Entry DOI: 10.7270/Q2542N79 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Mus musculus (mouse)) | BDBM27227
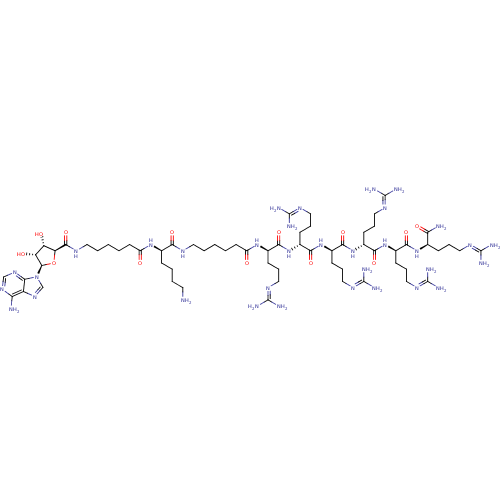
((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C64H118N34O13/c65-24-6-5-15-36(91-42(99)22-4-2-8-26-81-57(110)47-45(101)46(102)58(111-47)98-34-90-44-48(66)88-33-89-50(44)98)51(104)80-25-7-1-3-23-43(100)92-37(17-10-28-83-60(70)71)52(105)94-39(19-12-30-85-62(74)75)54(107)96-41(21-14-32-87-64(78)79)56(109)97-40(20-13-31-86-63(76)77)55(108)95-38(18-11-29-84-61(72)73)53(106)93-35(49(67)103)16-9-27-82-59(68)69/h33-41,45-47,58,101-102H,1-32,65H2,(H2,67,103)(H,80,104)(H,81,110)(H,91,99)(H,92,100)(H,93,106)(H,94,105)(H,95,108)(H,96,107)(H,97,109)(H2,66,88,89)(H4,68,69,82)(H4,70,71,83)(H4,72,73,84)(H4,74,75,85)(H4,76,77,86)(H4,78,79,87)/t35-,36-,37-,38-,39-,40-,41-,45+,46-,47+,58-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50311409

(CHEMBL1077375 | N-((6R,9R,12R,15R,18R,21R,31R)-1-a...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1ccc(s1)-c1ccnc(-[#7])n1)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H114N32O10S/c64-27-6-5-15-39(88-47(96)22-4-2-8-29-80-56(105)46-25-24-45(106-46)37-26-36-87-63(78)95-37)50(99)79-28-7-1-3-23-48(97)89-40(17-10-31-82-58(68)69)51(100)91-42(19-12-33-84-60(72)73)53(102)93-44(21-14-35-86-62(76)77)55(104)94-43(20-13-34-85-61(74)75)54(103)92-41(18-11-32-83-59(70)71)52(101)90-38(49(65)98)16-9-30-81-57(66)67/h24-26,36,38-44H,1-23,27-35,64H2,(H2,65,98)(H,79,99)(H,80,105)(H,88,96)(H,89,97)(H,90,101)(H,91,100)(H,92,103)(H,93,102)(H,94,104)(H4,66,67,81)(H4,68,69,82)(H4,70,71,83)(H4,72,73,84)(H4,74,75,85)(H4,76,77,86)(H2,78,87,95)/t38-,39-,40-,41-,42-,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of PKACalpha in presence of 1000 uM ATP |
Bioorg Med Chem Lett 19: 6098-101 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.026
BindingDB Entry DOI: 10.7270/Q2JM29R4 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM27249

(ARC-903 | N-[(1R)-4-carbamimidamido-1-{[(1R)-4-car...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C53H96N28O9S/c54-42(83)34(12-5-22-69-48(55)56)77-44(85)36(14-7-24-71-50(59)60)79-46(87)38(16-9-26-73-52(63)64)81-47(88)39(17-10-27-74-53(65)66)80-45(86)37(15-8-25-72-51(61)62)78-43(84)35(13-6-23-70-49(57)58)76-41(82)19-2-1-3-21-67-29-30-75-91(89,90)40-18-4-11-32-31-68-28-20-33(32)40/h4,11,18,20,28,31,34-39,67,75H,1-3,5-10,12-17,19,21-27,29-30H2,(H2,54,83)(H,76,82)(H,77,85)(H,78,84)(H,79,87)(H,80,86)(H,81,88)(H4,55,56,69)(H4,57,58,70)(H4,59,60,71)(H4,61,62,72)(H4,63,64,73)(H4,65,66,74)/t34-,35-,36-,37-,38-,39-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-583/ARC-1042/ARC-1059 from His6-tagged recombinant human p70S6K by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM27249

(ARC-903 | N-[(1R)-4-carbamimidamido-1-{[(1R)-4-car...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C53H96N28O9S/c54-42(83)34(12-5-22-69-48(55)56)77-44(85)36(14-7-24-71-50(59)60)79-46(87)38(16-9-26-73-52(63)64)81-47(88)39(17-10-27-74-53(65)66)80-45(86)37(15-8-25-72-51(61)62)78-43(84)35(13-6-23-70-49(57)58)76-41(82)19-2-1-3-21-67-29-30-75-91(89,90)40-18-4-11-32-31-68-28-20-33(32)40/h4,11,18,20,28,31,34-39,67,75H,1-3,5-10,12-17,19,21-27,29-30H2,(H2,54,83)(H,76,82)(H,77,85)(H,78,84)(H,79,87)(H,80,86)(H,81,88)(H4,55,56,69)(H4,57,58,70)(H4,59,60,71)(H4,61,62,72)(H4,63,64,73)(H4,65,66,74)/t34-,35-,36-,37-,38-,39-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-583/ARC-1042/ARC-1059 from His6-tagged recombinant human p70S6K by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM27248
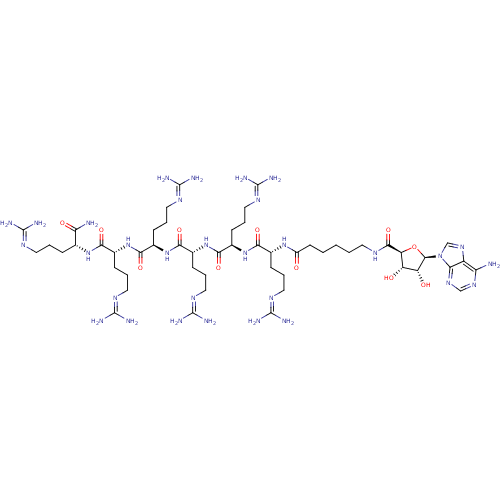
(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C52H95N31O11/c53-37-33-39(75-24-74-37)83(25-76-33)46-35(86)34(85)36(94-46)45(93)67-17-3-1-2-16-32(84)77-27(11-5-19-69-48(57)58)40(88)79-29(13-7-21-71-50(61)62)42(90)81-31(15-9-23-73-52(65)66)44(92)82-30(14-8-22-72-51(63)64)43(91)80-28(12-6-20-70-49(59)60)41(89)78-26(38(54)87)10-4-18-68-47(55)56/h24-31,34-36,46,85-86H,1-23H2,(H2,54,87)(H,67,93)(H,77,84)(H,78,89)(H,79,88)(H,80,91)(H,81,90)(H,82,92)(H2,53,74,75)(H4,55,56,68)(H4,57,58,69)(H4,59,60,70)(H4,61,62,71)(H4,63,64,72)(H4,65,66,73)/t26-,27-,28-,29-,30-,31-,34+,35-,36+,46-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic and Bioorganic Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory potency towards human cAPK C alpha in the presence of 100uM ATP and 30uM TAMRA-kemptide |
J Med Chem 49: 7150-9 (2006)
Article DOI: 10.1021/jm0605942
BindingDB Entry DOI: 10.7270/Q2542N79 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM27248

(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C52H95N31O11/c53-37-33-39(75-24-74-37)83(25-76-33)46-35(86)34(85)36(94-46)45(93)67-17-3-1-2-16-32(84)77-27(11-5-19-69-48(57)58)40(88)79-29(13-7-21-71-50(61)62)42(90)81-31(15-9-23-73-52(65)66)44(92)82-30(14-8-22-72-51(63)64)43(91)80-28(12-6-20-70-49(59)60)41(89)78-26(38(54)87)10-4-18-68-47(55)56/h24-31,34-36,46,85-86H,1-23H2,(H2,54,87)(H,67,93)(H,77,84)(H,78,89)(H,79,88)(H,80,91)(H,81,90)(H,82,92)(H2,53,74,75)(H4,55,56,68)(H4,57,58,69)(H4,59,60,70)(H4,61,62,71)(H4,63,64,72)(H4,65,66,73)/t26-,27-,28-,29-,30-,31-,34+,35-,36+,46-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic and Bioorganic Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory potency towards human cAPK C alpha in the presence of 100uM ATP and 30uM TAMRA-kemptide |
J Med Chem 49: 7150-9 (2006)
Article DOI: 10.1021/jm0605942
BindingDB Entry DOI: 10.7270/Q2542N79 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Mus musculus (mouse)) | BDBM27248

(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C52H95N31O11/c53-37-33-39(75-24-74-37)83(25-76-33)46-35(86)34(85)36(94-46)45(93)67-17-3-1-2-16-32(84)77-27(11-5-19-69-48(57)58)40(88)79-29(13-7-21-71-50(61)62)42(90)81-31(15-9-23-73-52(65)66)44(92)82-30(14-8-22-72-51(63)64)43(91)80-28(12-6-20-70-49(59)60)41(89)78-26(38(54)87)10-4-18-68-47(55)56/h24-31,34-36,46,85-86H,1-23H2,(H2,54,87)(H,67,93)(H,77,84)(H,78,89)(H,79,88)(H,80,91)(H,81,90)(H,82,92)(H2,53,74,75)(H4,55,56,68)(H4,57,58,69)(H4,59,60,70)(H4,61,62,71)(H4,63,64,72)(H4,65,66,73)/t26-,27-,28-,29-,30-,31-,34+,35-,36+,46-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50382219

(CHEMBL2023843)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#7](-[#6]-[#6]-1)S(=O)(=O)c1cccc2cnccc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C70H127N31O11S/c71-31-8-7-20-49(93-56(102)28-5-2-1-3-10-40-100-41-18-42-101(44-43-100)113(111,112)55-27-11-19-46-45-85-39-30-47(46)55)59(105)86-32-9-4-6-29-57(103)94-50(22-13-34-88-66(75)76)60(106)96-52(24-15-36-90-68(79)80)62(108)98-54(26-17-38-92-70(83)84)64(110)99-53(25-16-37-91-69(81)82)63(109)97-51(23-14-35-89-67(77)78)61(107)95-48(58(72)104)21-12-33-87-65(73)74/h11,19,27,30,39,45,48-54H,1-10,12-18,20-26,28-29,31-38,40-44,71H2,(H2,72,104)(H,86,105)(H,93,102)(H,94,103)(H,95,107)(H,96,106)(H,97,109)(H,98,108)(H,99,110)(H4,73,74,87)(H4,75,76,88)(H4,77,78,89)(H4,79,80,90)(H4,81,82,91)(H4,83,84,92)/t48-,49-,50-,51-,52-,53-,54-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-1063 from His6-tagged recombinant human ROCK2 by luminescence intensity assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50311409

(CHEMBL1077375 | N-((6R,9R,12R,15R,18R,21R,31R)-1-a...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1ccc(s1)-c1ccnc(-[#7])n1)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H114N32O10S/c64-27-6-5-15-39(88-47(96)22-4-2-8-29-80-56(105)46-25-24-45(106-46)37-26-36-87-63(78)95-37)50(99)79-28-7-1-3-23-48(97)89-40(17-10-31-82-58(68)69)51(100)91-42(19-12-33-84-60(72)73)53(102)93-44(21-14-35-86-62(76)77)55(104)94-43(20-13-34-85-61(74)75)54(103)92-41(18-11-32-83-59(70)71)52(101)90-38(49(65)98)16-9-30-81-57(66)67/h24-26,36,38-44H,1-23,27-35,64H2,(H2,65,98)(H,79,99)(H,80,105)(H,88,96)(H,89,97)(H,90,101)(H,91,100)(H,92,103)(H,93,102)(H,94,104)(H4,66,67,81)(H4,68,69,82)(H4,70,71,83)(H4,72,73,84)(H4,74,75,85)(H4,76,77,86)(H2,78,87,95)/t38-,39-,40-,41-,42-,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of PKBgamma in presence of 100 uM ATP |
Bioorg Med Chem Lett 19: 6098-101 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.026
BindingDB Entry DOI: 10.7270/Q2JM29R4 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50219803

(6-{[(1S,3R,4R)-3-(6-amino-9H-purin-9-yl)-4-hydroxy...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6@@H](-[#8])-[#6@@H](-[#6]-1)-n1cnc2c(-[#7])ncnc12)-[#6](-[#7])=O Show InChI InChI=1S/C53H97N31O9/c54-39-38-41(76-26-75-39)84(27-77-38)35-24-28(25-36(35)85)42(88)68-17-3-1-2-16-37(86)78-30(11-5-19-70-49(58)59)43(89)80-32(13-7-21-72-51(62)63)45(91)82-34(15-9-23-74-53(66)67)47(93)83-33(14-8-22-73-52(64)65)46(92)81-31(12-6-20-71-50(60)61)44(90)79-29(40(55)87)10-4-18-69-48(56)57/h26-36,85H,1-25H2,(H2,55,87)(H,68,88)(H,78,86)(H,79,90)(H,80,89)(H,81,92)(H,82,91)(H,83,93)(H2,54,75,76)(H4,56,57,69)(H4,58,59,70)(H4,60,61,71)(H4,62,63,72)(H4,64,65,73)(H4,66,67,74)/t28-,29-,30-,31-,32-,33-,34-,35+,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of cAPK Calpha in presence of 0.1 mM ATP |
Bioorg Med Chem Lett 17: 5336-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.016
BindingDB Entry DOI: 10.7270/Q2FQ9WBJ |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Mus musculus (mouse)) | BDBM27226
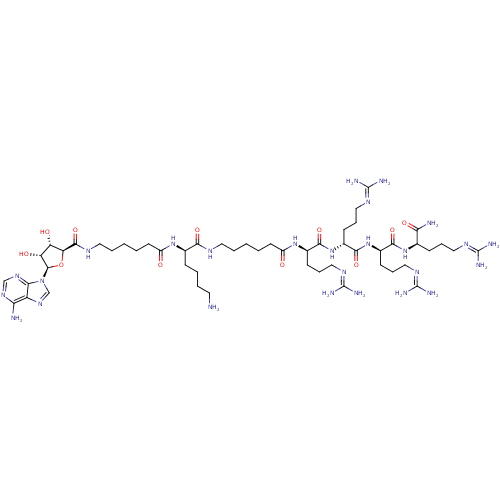
((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C52H94N26O11/c53-20-6-5-13-30(73-34(79)18-4-2-8-22-65-47(88)39-37(81)38(82)48(89-39)78-28-72-36-40(54)70-27-71-42(36)78)43(84)64-21-7-1-3-19-35(80)74-31(15-10-24-67-50(58)59)44(85)76-33(17-12-26-69-52(62)63)46(87)77-32(16-11-25-68-51(60)61)45(86)75-29(41(55)83)14-9-23-66-49(56)57/h27-33,37-39,48,81-82H,1-26,53H2,(H2,55,83)(H,64,84)(H,65,88)(H,73,79)(H,74,80)(H,75,86)(H,76,85)(H,77,87)(H2,54,70,71)(H4,56,57,66)(H4,58,59,67)(H4,60,61,68)(H4,62,63,69)/t29-,30-,31-,32-,33-,37+,38-,39+,48-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50311405
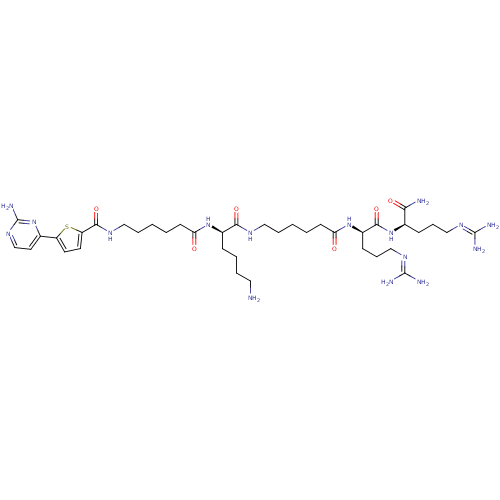
(CHEMBL1077369 | N-((6R,9R,19R)-1-amino-19-(4-amino...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1ccc(s1)-c1ccnc(-[#7])n1)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C39H66N16O6S/c40-19-6-5-11-27(52-31(56)14-4-2-8-21-48-36(61)30-17-16-29(62-30)25-18-24-51-39(46)55-25)34(59)47-20-7-1-3-15-32(57)53-28(13-10-23-50-38(44)45)35(60)54-26(33(41)58)12-9-22-49-37(42)43/h16-18,24,26-28H,1-15,19-23,40H2,(H2,41,58)(H,47,59)(H,48,61)(H,52,56)(H,53,57)(H,54,60)(H4,42,43,49)(H4,44,45,50)(H2,46,51,55)/t26-,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of PKACalpha in presense of 100 uM ATP |
Bioorg Med Chem Lett 19: 6098-101 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.026
BindingDB Entry DOI: 10.7270/Q2JM29R4 |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM27227

((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C64H118N34O13/c65-24-6-5-15-36(91-42(99)22-4-2-8-26-81-57(110)47-45(101)46(102)58(111-47)98-34-90-44-48(66)88-33-89-50(44)98)51(104)80-25-7-1-3-23-43(100)92-37(17-10-28-83-60(70)71)52(105)94-39(19-12-30-85-62(74)75)54(107)96-41(21-14-32-87-64(78)79)56(109)97-40(20-13-31-86-63(76)77)55(108)95-38(18-11-29-84-61(72)73)53(106)93-35(49(67)103)16-9-27-82-59(68)69/h33-41,45-47,58,101-102H,1-32,65H2,(H2,67,103)(H,80,104)(H,81,110)(H,91,99)(H,92,100)(H,93,106)(H,94,105)(H,95,108)(H,96,107)(H,97,109)(H2,66,88,89)(H4,68,69,82)(H4,70,71,83)(H4,72,73,84)(H4,74,75,85)(H4,76,77,86)(H4,78,79,87)/t35-,36-,37-,38-,39-,40-,41-,45+,46-,47+,58-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50382216
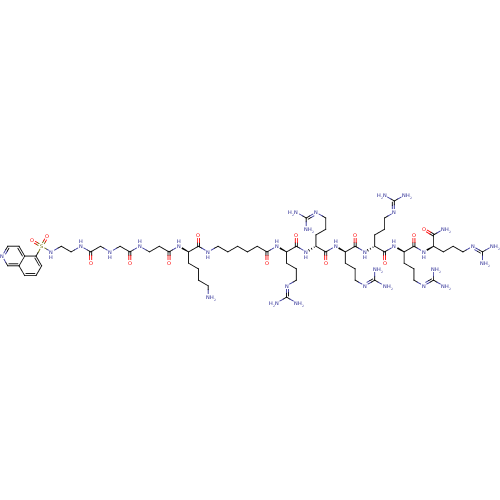
(CHEMBL2023839)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C66H119N33O13S/c67-25-4-3-14-43(93-51(101)24-34-83-52(102)38-82-39-53(103)84-35-36-92-113(111,112)49-21-6-13-40-37-81-33-23-41(40)49)55(105)85-26-5-1-2-22-50(100)94-44(16-8-28-87-62(71)72)56(106)96-46(18-10-30-89-64(75)76)58(108)98-48(20-12-32-91-66(79)80)60(110)99-47(19-11-31-90-65(77)78)59(109)97-45(17-9-29-88-63(73)74)57(107)95-42(54(68)104)15-7-27-86-61(69)70/h6,13,21,23,33,37,42-48,82,92H,1-5,7-12,14-20,22,24-32,34-36,38-39,67H2,(H2,68,104)(H,83,102)(H,84,103)(H,85,105)(H,93,101)(H,94,100)(H,95,107)(H,96,106)(H,97,109)(H,98,108)(H,99,110)(H4,69,70,86)(H4,71,72,87)(H4,73,74,88)(H4,75,76,89)(H4,77,78,90)(H4,79,80,91)/t42-,43-,44-,45-,46-,47-,48-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-1042 from His6-tagged recombinant human ROCK2 by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Mus musculus (mouse)) | BDBM27229
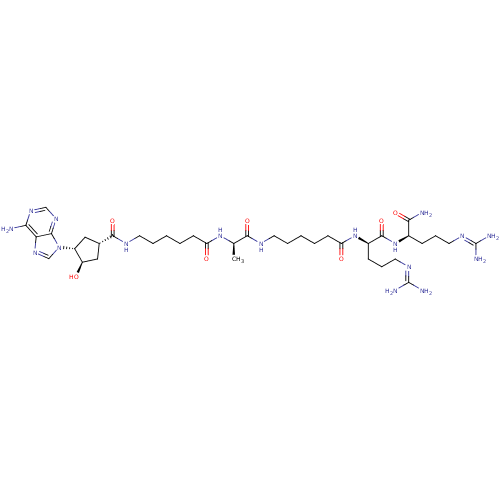
(6-{[(1S,3R,4R)-3-(6-amino-9H-purin-9-yl)-4-hydroxy...)Show SMILES [#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6@@H](-[#8])-[#6@@H](-[#6]-1)-n1cnc2c(-[#7])ncnc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C38H65N17O7/c1-22(52-28(57)12-4-2-7-15-46-35(61)23-18-26(27(56)19-23)55-21-51-30-31(39)49-20-50-33(30)55)34(60)45-14-6-3-5-13-29(58)53-25(11-9-17-48-38(43)44)36(62)54-24(32(40)59)10-8-16-47-37(41)42/h20-27,56H,2-19H2,1H3,(H2,40,59)(H,45,60)(H,46,61)(H,52,57)(H,53,58)(H,54,62)(H2,39,49,50)(H4,41,42,47)(H4,43,44,48)/t22-,23+,24-,25-,26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM27249

(ARC-903 | N-[(1R)-4-carbamimidamido-1-{[(1R)-4-car...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C53H96N28O9S/c54-42(83)34(12-5-22-69-48(55)56)77-44(85)36(14-7-24-71-50(59)60)79-46(87)38(16-9-26-73-52(63)64)81-47(88)39(17-10-27-74-53(65)66)80-45(86)37(15-8-25-72-51(61)62)78-43(84)35(13-6-23-70-49(57)58)76-41(82)19-2-1-3-21-67-29-30-75-91(89,90)40-18-4-11-32-31-68-28-20-33(32)40/h4,11,18,20,28,31,34-39,67,75H,1-3,5-10,12-17,19,21-27,29-30H2,(H2,54,83)(H,76,82)(H,77,85)(H,78,84)(H,79,87)(H,80,86)(H,81,88)(H4,55,56,69)(H4,57,58,70)(H4,59,60,71)(H4,61,62,72)(H4,63,64,73)(H4,65,66,74)/t34-,35-,36-,37-,38-,39-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50382222
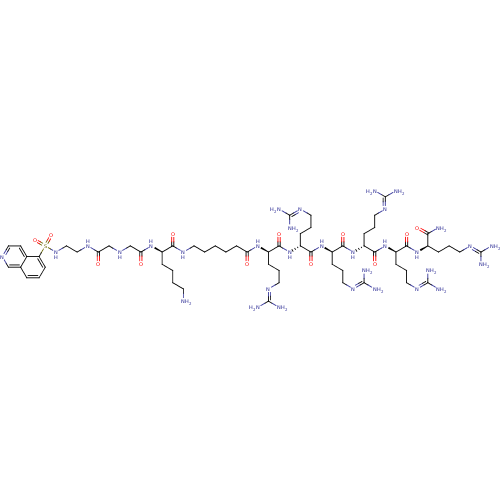
(CHEMBL2023838)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H114N32O12S/c64-24-4-3-14-41(90-50(98)37-79-36-49(97)80-33-34-88-108(106,107)47-21-6-13-38-35-78-32-23-39(38)47)52(100)81-25-5-1-2-22-48(96)89-42(16-8-27-83-59(68)69)53(101)92-44(18-10-29-85-61(72)73)55(103)94-46(20-12-31-87-63(76)77)57(105)95-45(19-11-30-86-62(74)75)56(104)93-43(17-9-28-84-60(70)71)54(102)91-40(51(65)99)15-7-26-82-58(66)67/h6,13,21,23,32,35,40-46,79,88H,1-5,7-12,14-20,22,24-31,33-34,36-37,64H2,(H2,65,99)(H,80,97)(H,81,100)(H,89,96)(H,90,98)(H,91,102)(H,92,101)(H,93,104)(H,94,103)(H,95,105)(H4,66,67,82)(H4,68,69,83)(H4,70,71,84)(H4,72,73,85)(H4,74,75,86)(H4,76,77,87)/t40-,41-,42-,43-,44-,45-,46-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-1042 from His6-tagged recombinant human ROCK2 by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM27248

(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C52H95N31O11/c53-37-33-39(75-24-74-37)83(25-76-33)46-35(86)34(85)36(94-46)45(93)67-17-3-1-2-16-32(84)77-27(11-5-19-69-48(57)58)40(88)79-29(13-7-21-71-50(61)62)42(90)81-31(15-9-23-73-52(65)66)44(92)82-30(14-8-22-72-51(63)64)43(91)80-28(12-6-20-70-49(59)60)41(89)78-26(38(54)87)10-4-18-68-47(55)56/h24-31,34-36,46,85-86H,1-23H2,(H2,54,87)(H,67,93)(H,77,84)(H,78,89)(H,79,88)(H,80,91)(H,81,90)(H,82,92)(H2,53,74,75)(H4,55,56,68)(H4,57,58,69)(H4,59,60,70)(H4,61,62,71)(H4,63,64,72)(H4,65,66,73)/t26-,27-,28-,29-,30-,31-,34+,35-,36+,46-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50311410

(CHEMBL1077376 | N-((5S,8R,11R,14R,17R,20R,23R,33R)...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1ccc(s1)-c1ccnc(-[#7])n1)-[#6](-[#7])=O |r| Show InChI InChI=1S/C69H126N34O11S/c70-30-7-5-17-42(54(72)106)97-57(109)45(20-12-35-89-64(75)76)99-59(111)47(22-14-37-91-66(79)80)101-61(113)49(24-16-39-93-68(83)84)102-60(112)48(23-15-38-92-67(81)82)100-58(110)46(21-13-36-90-65(77)78)98-56(108)44(19-11-34-88-63(73)74)96-53(105)26-3-1-9-32-86-55(107)43(18-6-8-31-71)95-52(104)25-4-2-10-33-87-62(114)51-28-27-50(115-51)41-29-40-94-69(85)103-41/h27-29,40,42-49H,1-26,30-39,70-71H2,(H2,72,106)(H,86,107)(H,87,114)(H,95,104)(H,96,105)(H,97,109)(H,98,108)(H,99,111)(H,100,110)(H,101,113)(H,102,112)(H4,73,74,88)(H4,75,76,89)(H4,77,78,90)(H4,79,80,91)(H4,81,82,92)(H4,83,84,93)(H2,85,94,103)/t42-,43+,44+,45+,46+,47+,48+,49+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of PKBgamma in presence of 100 uM ATP |
Bioorg Med Chem Lett 19: 6098-101 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.026
BindingDB Entry DOI: 10.7270/Q2JM29R4 |
More data for this
Ligand-Target Pair | |
cGMP-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM27249

(ARC-903 | N-[(1R)-4-carbamimidamido-1-{[(1R)-4-car...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C53H96N28O9S/c54-42(83)34(12-5-22-69-48(55)56)77-44(85)36(14-7-24-71-50(59)60)79-46(87)38(16-9-26-73-52(63)64)81-47(88)39(17-10-27-74-53(65)66)80-45(86)37(15-8-25-72-51(61)62)78-43(84)35(13-6-23-70-49(57)58)76-41(82)19-2-1-3-21-67-29-30-75-91(89,90)40-18-4-11-32-31-68-28-20-33(32)40/h4,11,18,20,28,31,34-39,67,75H,1-3,5-10,12-17,19,21-27,29-30H2,(H2,54,83)(H,76,82)(H,77,85)(H,78,84)(H,79,87)(H,80,86)(H,81,88)(H4,55,56,69)(H4,57,58,70)(H4,59,60,71)(H4,61,62,72)(H4,63,64,73)(H4,65,66,74)/t34-,35-,36-,37-,38-,39-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-583/ARC-1042/ARC-1059 from His6-tagged recombinant human PKG1alpha by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM27250

(6-{[(1S,3R,4R)-3-(6-amino-9H-purin-9-yl)-4-hydroxy...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6@@H](-[#8])-[#6@@H](-[#6]-1)-n1cnc2c(-[#7])ncnc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C53H97N31O9/c54-39-38-41(76-26-75-39)84(27-77-38)35-24-28(25-36(35)85)42(88)68-17-3-1-2-16-37(86)78-30(11-5-19-70-49(58)59)43(89)80-32(13-7-21-72-51(62)63)45(91)82-34(15-9-23-74-53(66)67)47(93)83-33(14-8-22-73-52(64)65)46(92)81-31(12-6-20-71-50(60)61)44(90)79-29(40(55)87)10-4-18-69-48(56)57/h26-36,85H,1-25H2,(H2,55,87)(H,68,88)(H,78,86)(H,79,90)(H,80,89)(H,81,92)(H,82,91)(H,83,93)(H2,54,75,76)(H4,56,57,69)(H4,58,59,70)(H4,60,61,71)(H4,62,63,72)(H4,64,65,73)(H4,66,67,74)/t28-,29+,30+,31+,32+,33+,34+,35+,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM27225

((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C40H70N18O9/c41-16-6-5-11-24(55-26(59)14-4-2-8-18-49-37(66)31-29(61)30(62)38(67-31)58-22-54-28-32(42)52-21-53-34(28)58)35(64)48-17-7-1-3-15-27(60)56-25(13-10-20-51-40(46)47)36(65)57-23(33(43)63)12-9-19-50-39(44)45/h21-25,29-31,38,61-62H,1-20,41H2,(H2,43,63)(H,48,64)(H,49,66)(H,55,59)(H,56,60)(H,57,65)(H2,42,52,53)(H4,44,45,50)(H4,46,47,51)/t23-,24-,25-,29+,30-,31+,38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of PKACalpha in presense of 100 uM ATP |
Bioorg Med Chem Lett 19: 6098-101 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.026
BindingDB Entry DOI: 10.7270/Q2JM29R4 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Mus musculus (mouse)) | BDBM27225

((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C40H70N18O9/c41-16-6-5-11-24(55-26(59)14-4-2-8-18-49-37(66)31-29(61)30(62)38(67-31)58-22-54-28-32(42)52-21-53-34(28)58)35(64)48-17-7-1-3-15-27(60)56-25(13-10-20-51-40(46)47)36(65)57-23(33(43)63)12-9-19-50-39(44)45/h21-25,29-31,38,61-62H,1-20,41H2,(H2,43,63)(H,48,64)(H,49,66)(H,55,59)(H,56,60)(H,57,65)(H2,42,52,53)(H4,44,45,50)(H4,46,47,51)/t23-,24-,25-,29+,30-,31+,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM27226

((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C52H94N26O11/c53-20-6-5-13-30(73-34(79)18-4-2-8-22-65-47(88)39-37(81)38(82)48(89-39)78-28-72-36-40(54)70-27-71-42(36)78)43(84)64-21-7-1-3-19-35(80)74-31(15-10-24-67-50(58)59)44(85)76-33(17-12-26-69-52(62)63)46(87)77-32(16-11-25-68-51(60)61)45(86)75-29(41(55)83)14-9-23-66-49(56)57/h27-33,37-39,48,81-82H,1-26,53H2,(H2,55,83)(H,64,84)(H,65,88)(H,73,79)(H,74,80)(H,75,86)(H,76,85)(H,77,87)(H2,54,70,71)(H4,56,57,66)(H4,58,59,67)(H4,60,61,68)(H4,62,63,69)/t29-,30-,31-,32-,33-,37+,38-,39+,48-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50311407

(CHEMBL1077371 | N-((6R,9R,19R)-1-amino-19-(4-amino...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1cccc(c1)-c1ccnc(-[#7])n1)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C41H68N16O6/c42-20-6-5-14-31(54-33(58)17-3-1-7-21-49-36(61)28-13-9-12-27(26-28)29-19-25-53-41(48)57-29)37(62)50-22-8-2-4-18-34(59)55-32(16-11-24-52-40(46)47)38(63)56-30(35(43)60)15-10-23-51-39(44)45/h9,12-13,19,25-26,30-32H,1-8,10-11,14-18,20-24,42H2,(H2,43,60)(H,49,61)(H,50,62)(H,54,58)(H,55,59)(H,56,63)(H4,44,45,51)(H4,46,47,52)(H2,48,53,57)/t30-,31-,32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of PKACalpha in presense of 100 uM ATP |
Bioorg Med Chem Lett 19: 6098-101 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.026
BindingDB Entry DOI: 10.7270/Q2JM29R4 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM27249

(ARC-903 | N-[(1R)-4-carbamimidamido-1-{[(1R)-4-car...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C53H96N28O9S/c54-42(83)34(12-5-22-69-48(55)56)77-44(85)36(14-7-24-71-50(59)60)79-46(87)38(16-9-26-73-52(63)64)81-47(88)39(17-10-27-74-53(65)66)80-45(86)37(15-8-25-72-51(61)62)78-43(84)35(13-6-23-70-49(57)58)76-41(82)19-2-1-3-21-67-29-30-75-91(89,90)40-18-4-11-32-31-68-28-20-33(32)40/h4,11,18,20,28,31,34-39,67,75H,1-3,5-10,12-17,19,21-27,29-30H2,(H2,54,83)(H,76,82)(H,77,85)(H,78,84)(H,79,87)(H,80,86)(H,81,88)(H4,55,56,69)(H4,57,58,70)(H4,59,60,71)(H4,61,62,72)(H4,63,64,73)(H4,65,66,74)/t34-,35-,36-,37-,38-,39-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-583/ARC-1042/ARC-1059 from His6-tagged recombinant human MSK1 by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM27249

(ARC-903 | N-[(1R)-4-carbamimidamido-1-{[(1R)-4-car...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C53H96N28O9S/c54-42(83)34(12-5-22-69-48(55)56)77-44(85)36(14-7-24-71-50(59)60)79-46(87)38(16-9-26-73-52(63)64)81-47(88)39(17-10-27-74-53(65)66)80-45(86)37(15-8-25-72-51(61)62)78-43(84)35(13-6-23-70-49(57)58)76-41(82)19-2-1-3-21-67-29-30-75-91(89,90)40-18-4-11-32-31-68-28-20-33(32)40/h4,11,18,20,28,31,34-39,67,75H,1-3,5-10,12-17,19,21-27,29-30H2,(H2,54,83)(H,76,82)(H,77,85)(H,78,84)(H,79,87)(H,80,86)(H,81,88)(H4,55,56,69)(H4,57,58,70)(H4,59,60,71)(H4,61,62,72)(H4,63,64,73)(H4,65,66,74)/t34-,35-,36-,37-,38-,39-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-583/ARC-1042/ARC-1059 from His6-tagged recombinant human MSK1 by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50311405

(CHEMBL1077369 | N-((6R,9R,19R)-1-amino-19-(4-amino...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1ccc(s1)-c1ccnc(-[#7])n1)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C39H66N16O6S/c40-19-6-5-11-27(52-31(56)14-4-2-8-21-48-36(61)30-17-16-29(62-30)25-18-24-51-39(46)55-25)34(59)47-20-7-1-3-15-32(57)53-28(13-10-23-50-38(44)45)35(60)54-26(33(41)58)12-9-22-49-37(42)43/h16-18,24,26-28H,1-15,19-23,40H2,(H2,41,58)(H,47,59)(H,48,61)(H,52,56)(H,53,57)(H,54,60)(H4,42,43,49)(H4,44,45,50)(H2,46,51,55)/t26-,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of PKACalpha in presence of 1000 uM ATP |
Bioorg Med Chem Lett 19: 6098-101 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.026
BindingDB Entry DOI: 10.7270/Q2JM29R4 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Mus musculus (mouse)) | BDBM27228

(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...)Show SMILES [#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C37H63N17O9/c1-20(51-23(55)12-4-2-7-15-45-34(62)28-26(57)27(58)35(63-28)54-19-50-25-29(38)48-18-49-31(25)54)32(60)44-14-6-3-5-13-24(56)52-22(11-9-17-47-37(42)43)33(61)53-21(30(39)59)10-8-16-46-36(40)41/h18-22,26-28,35,57-58H,2-17H2,1H3,(H2,39,59)(H,44,60)(H,45,62)(H,51,55)(H,52,56)(H,53,61)(H2,38,48,49)(H4,40,41,46)(H4,42,43,47)/t20-,21-,22-,26+,27-,28+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50219805

(6-{[(1R,3S,4S)-3-(6-amino-9H-purin-9-yl)-4-hydroxy...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#6]-[#6@H](-[#8])-[#6@H](-[#6]-1)-n1cnc2c(-[#7])ncnc12)-[#6](-[#7])=O Show InChI InChI=1S/C53H97N31O9/c54-39-38-41(76-26-75-39)84(27-77-38)35-24-28(25-36(35)85)42(88)68-17-3-1-2-16-37(86)78-30(11-5-19-70-49(58)59)43(89)80-32(13-7-21-72-51(62)63)45(91)82-34(15-9-23-74-53(66)67)47(93)83-33(14-8-22-73-52(64)65)46(92)81-31(12-6-20-71-50(60)61)44(90)79-29(40(55)87)10-4-18-69-48(56)57/h26-36,85H,1-25H2,(H2,55,87)(H,68,88)(H,78,86)(H,79,90)(H,80,89)(H,81,92)(H,82,91)(H,83,93)(H2,54,75,76)(H4,56,57,69)(H4,58,59,70)(H4,60,61,71)(H4,62,63,72)(H4,64,65,73)(H4,66,67,74)/t28-,29+,30+,31+,32+,33+,34+,35+,36+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97.3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of cAPK Calpha in presence of 0.1 mM ATP |
Bioorg Med Chem Lett 17: 5336-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.016
BindingDB Entry DOI: 10.7270/Q2FQ9WBJ |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Mus musculus (mouse)) | BDBM15211
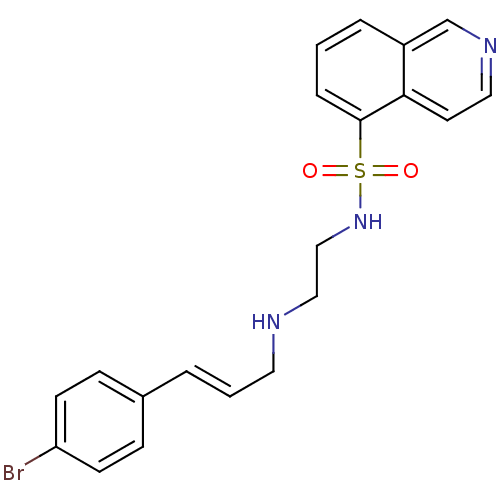
(CHEMBL104264 | H-89 | H89 | HT-89 (H-89) | N-(2-{[...)Show SMILES Brc1ccc(\C=C\CNCCNS(=O)(=O)c2cccc3cnccc23)cc1 Show InChI InChI=1S/C20H20BrN3O2S/c21-18-8-6-16(7-9-18)3-2-11-22-13-14-24-27(25,26)20-5-1-4-17-15-23-12-10-19(17)20/h1-10,12,15,22,24H,11,13-14H2/b3-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry in Estonia
| Assay Description
The IC50 values of the inhibitors corresponding to the concentration of the inhibitor decreasing the enzyme activity 50% were measured using fluoresc... |
J Med Chem 52: 308-21 (2009)
Article DOI: 10.1021/jm800797n
BindingDB Entry DOI: 10.7270/Q2RX99FQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50366588
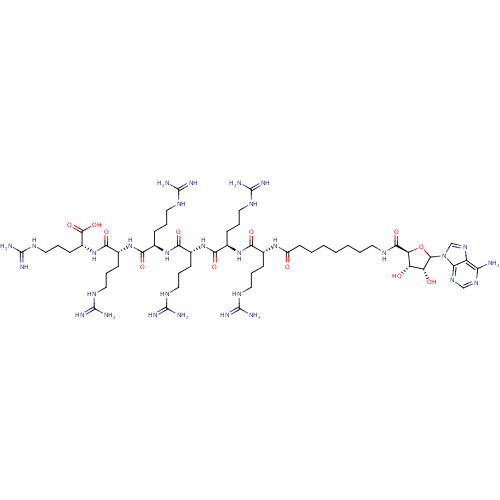
(CHEMBL610876)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCNC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C54H98N30O12/c55-39-35-40(76-26-75-39)84(27-77-35)47-37(87)36(86)38(96-47)46(93)68-19-5-3-1-2-4-18-34(85)78-28(12-6-20-69-49(56)57)41(88)79-29(13-7-21-70-50(58)59)42(89)80-30(14-8-22-71-51(60)61)43(90)81-31(15-9-23-72-52(62)63)44(91)82-32(16-10-24-73-53(64)65)45(92)83-33(48(94)95)17-11-25-74-54(66)67/h26-33,36-38,47,86-87H,1-25H2,(H,68,93)(H,78,85)(H,79,88)(H,80,89)(H,81,90)(H,82,91)(H,83,92)(H,94,95)(H2,55,75,76)(H4,56,57,69)(H4,58,59,70)(H4,60,61,71)(H4,62,63,72)(H4,64,65,73)(H4,66,67,74)/t28-,29-,30-,31-,32-,33-,36+,37-,38+,47?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Tartu University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein kinase C beta isoform (PKC) from pig spleen. |
Bioorg Med Chem Lett 9: 1447-52 (1999)
BindingDB Entry DOI: 10.7270/Q2RF5VJB |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50382223

(CHEMBL2023840)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C51H91N29O10S/c52-40(83)31(9-2-17-68-46(53)54)76-42(85)33(11-4-19-70-48(57)58)78-44(87)35(13-6-21-72-50(61)62)80-45(88)36(14-7-22-73-51(63)64)79-43(86)34(12-5-20-71-49(59)60)77-41(84)32(10-3-18-69-47(55)56)75-39(82)28-66-27-38(81)67-24-25-74-91(89,90)37-15-1-8-29-26-65-23-16-30(29)37/h1,8,15-16,23,26,31-36,66,74H,2-7,9-14,17-22,24-25,27-28H2,(H2,52,83)(H,67,81)(H,75,82)(H,76,85)(H,77,84)(H,78,87)(H,79,86)(H,80,88)(H4,53,54,68)(H4,55,56,69)(H4,57,58,70)(H4,59,60,71)(H4,61,62,72)(H4,63,64,73)/t31-,32-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-1042 from His6-tagged recombinant human ROCK2 by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50382223

(CHEMBL2023840)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c1cccc2cnccc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C51H91N29O10S/c52-40(83)31(9-2-17-68-46(53)54)76-42(85)33(11-4-19-70-48(57)58)78-44(87)35(13-6-21-72-50(61)62)80-45(88)36(14-7-22-73-51(63)64)79-43(86)34(12-5-20-71-49(59)60)77-41(84)32(10-3-18-69-47(55)56)75-39(82)28-66-27-38(81)67-24-25-74-91(89,90)37-15-1-8-29-26-65-23-16-30(29)37/h1,8,15-16,23,26,31-36,66,74H,2-7,9-14,17-22,24-25,27-28H2,(H2,52,83)(H,67,81)(H,75,82)(H,76,85)(H,77,84)(H,78,87)(H,79,86)(H,80,88)(H4,53,54,68)(H4,55,56,69)(H4,57,58,70)(H4,59,60,71)(H4,61,62,72)(H4,63,64,73)/t31-,32-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-1042 from His6-tagged recombinant human ROCK2 by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM27225

((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C40H70N18O9/c41-16-6-5-11-24(55-26(59)14-4-2-8-18-49-37(66)31-29(61)30(62)38(67-31)58-22-54-28-32(42)52-21-53-34(28)58)35(64)48-17-7-1-3-15-27(60)56-25(13-10-20-51-40(46)47)36(65)57-23(33(43)63)12-9-19-50-39(44)45/h21-25,29-31,38,61-62H,1-20,41H2,(H2,43,63)(H,48,64)(H,49,66)(H,55,59)(H,56,60)(H,57,65)(H2,42,52,53)(H4,44,45,50)(H4,46,47,51)/t23-,24-,25-,29+,30-,31+,38-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-1042 from His6-tagged recombinant human ROCK2 by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM27225

((2R)-6-amino-2-(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-pu...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C40H70N18O9/c41-16-6-5-11-24(55-26(59)14-4-2-8-18-49-37(66)31-29(61)30(62)38(67-31)58-22-54-28-32(42)52-21-53-34(28)58)35(64)48-17-7-1-3-15-27(60)56-25(13-10-20-51-40(46)47)36(65)57-23(33(43)63)12-9-19-50-39(44)45/h21-25,29-31,38,61-62H,1-20,41H2,(H2,43,63)(H,48,64)(H,49,66)(H,55,59)(H,56,60)(H,57,65)(H2,42,52,53)(H4,44,45,50)(H4,46,47,51)/t23-,24-,25-,29+,30-,31+,38-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-1042 from His6-tagged recombinant human ROCK2 by fluorescence anisotropy assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM27248

(6-{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-di...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1cnc2c(-[#7])ncnc12)-[#6](-[#7])=O |r| Show InChI InChI=1S/C52H95N31O11/c53-37-33-39(75-24-74-37)83(25-76-33)46-35(86)34(85)36(94-46)45(93)67-17-3-1-2-16-32(84)77-27(11-5-19-69-48(57)58)40(88)79-29(13-7-21-71-50(61)62)42(90)81-31(15-9-23-73-52(65)66)44(92)82-30(14-8-22-72-51(63)64)43(91)80-28(12-6-20-70-49(59)60)41(89)78-26(38(54)87)10-4-18-68-47(55)56/h24-31,34-36,46,85-86H,1-23H2,(H2,54,87)(H,67,93)(H,77,84)(H,78,89)(H,79,88)(H,80,91)(H,81,90)(H,82,92)(H2,53,74,75)(H4,55,56,68)(H4,57,58,69)(H4,59,60,70)(H4,61,62,71)(H4,63,64,72)(H4,65,66,73)/t26-,27-,28-,29-,30-,31-,34+,35-,36+,46-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-ARC-1063 from His6-tagged recombinant human ROCK2 by luminescence intensity assay |
Bioorg Med Chem Lett 22: 3425-30 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.101
BindingDB Entry DOI: 10.7270/Q2GT5P5N |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50311405

(CHEMBL1077369 | N-((6R,9R,19R)-1-amino-19-(4-amino...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1ccc(s1)-c1ccnc(-[#7])n1)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C39H66N16O6S/c40-19-6-5-11-27(52-31(56)14-4-2-8-21-48-36(61)30-17-16-29(62-30)25-18-24-51-39(46)55-25)34(59)47-20-7-1-3-15-32(57)53-28(13-10-23-50-38(44)45)35(60)54-26(33(41)58)12-9-22-49-37(42)43/h16-18,24,26-28H,1-15,19-23,40H2,(H2,41,58)(H,47,59)(H,48,61)(H,52,56)(H,53,57)(H,54,60)(H4,42,43,49)(H4,44,45,50)(H2,46,51,55)/t26-,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of PKBgamma in presence of 100 uM ATP |
Bioorg Med Chem Lett 19: 6098-101 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.026
BindingDB Entry DOI: 10.7270/Q2JM29R4 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50366583

(CHEMBL610874)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCNC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C52H94N30O12/c53-37-33-38(74-24-73-37)82(25-75-33)45-35(85)34(84)36(94-45)44(91)66-17-3-1-2-16-32(83)76-26(10-4-18-67-47(54)55)39(86)77-27(11-5-19-68-48(56)57)40(87)78-28(12-6-20-69-49(58)59)41(88)79-29(13-7-21-70-50(60)61)42(89)80-30(14-8-22-71-51(62)63)43(90)81-31(46(92)93)15-9-23-72-52(64)65/h24-31,34-36,45,84-85H,1-23H2,(H,66,91)(H,76,83)(H,77,86)(H,78,87)(H,79,88)(H,80,89)(H,81,90)(H,92,93)(H2,53,73,74)(H4,54,55,67)(H4,56,57,68)(H4,58,59,69)(H4,60,61,70)(H4,62,63,71)(H4,64,65,72)/t26-,27-,28-,29-,30-,31-,34+,35-,36+,45?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Tartu University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein kinase C beta isoform (PKC) from pig spleen. |
Bioorg Med Chem Lett 9: 1447-52 (1999)
BindingDB Entry DOI: 10.7270/Q2RF5VJB |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50366590
(CHEMBL611121)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCCCCNC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C57H104N30O12/c58-42-38-43(79-29-78-42)87(30-80-38)50-40(90)39(89)41(99-50)49(96)71-22-8-6-4-2-1-3-5-7-21-37(88)81-31(15-9-23-72-52(59)60)44(91)82-32(16-10-24-73-53(61)62)45(92)83-33(17-11-25-74-54(63)64)46(93)84-34(18-12-26-75-55(65)66)47(94)85-35(19-13-27-76-56(67)68)48(95)86-36(51(97)98)20-14-28-77-57(69)70/h29-36,39-41,50,89-90H,1-28H2,(H,71,96)(H,81,88)(H,82,91)(H,83,92)(H,84,93)(H,85,94)(H,86,95)(H,97,98)(H2,58,78,79)(H4,59,60,72)(H4,61,62,73)(H4,63,64,74)(H4,65,66,75)(H4,67,68,76)(H4,69,70,77)/t31-,32-,33-,34-,35-,36-,39+,40-,41+,50?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Tartu University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein kinase C beta isoform (PKC) from pig spleen. |
Bioorg Med Chem Lett 9: 1447-52 (1999)
BindingDB Entry DOI: 10.7270/Q2RF5VJB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data