 Found 187 hits with Last Name = 'rami' and Initial = 'h'
Found 187 hits with Last Name = 'rami' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412114
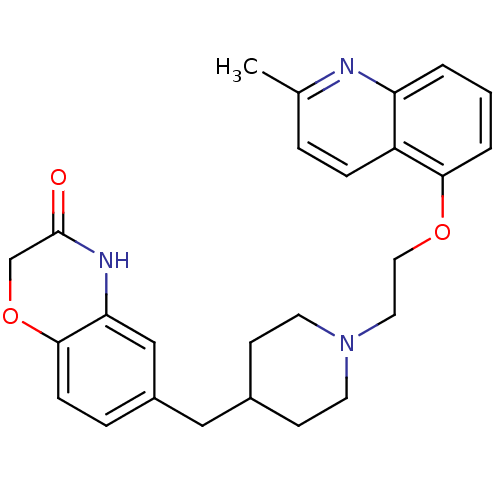
(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287729

(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show SMILES CN(CCOc1ccc(CC(Oc2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C25H24N2O5/c1-27(25-26-21-9-5-6-10-22(21)32-25)15-16-30-19-13-11-18(12-14-19)17-23(24(28)29)31-20-7-3-2-4-8-20/h2-14,23H,15-17H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50052204
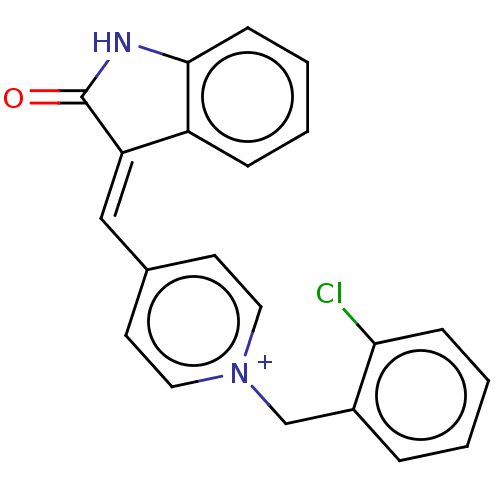
(CHEMBL3318392)Show SMILES [Cl-].Clc1ccccc1C[n+]1ccc(\C=C2\C(=O)Nc3ccccc23)cc1 Show InChI InChI=1S/C21H15ClN2O/c22-19-7-3-1-5-16(19)14-24-11-9-15(10-12-24)13-18-17-6-2-4-8-20(17)23-21(18)25/h1-13H,14H2/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of electric eel AChE using acetylthiocholine iodide substrate by Lineweaver-Burk plot analysis |
Eur J Med Chem 84: 375-81 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.017
BindingDB Entry DOI: 10.7270/Q2H996T1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474976

(CHEMBL180557)Show SMILES O=C1COc2ccc(CC3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C25H27N3O3/c29-25-17-31-24-7-6-19(16-22(24)27-25)15-18-8-11-28(12-9-18)13-14-30-23-5-1-4-21-20(23)3-2-10-26-21/h1-7,10,16,18H,8-9,11-15,17H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474983
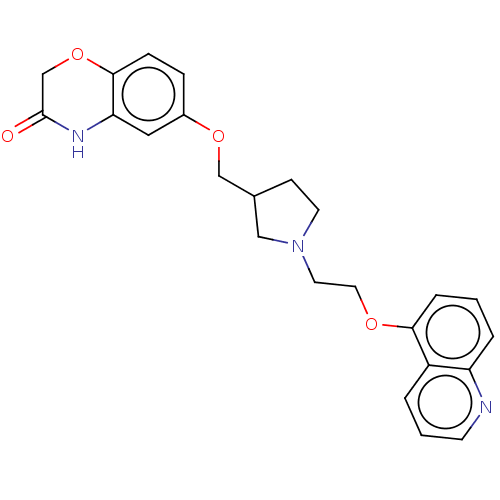
(CHEMBL183034)Show SMILES O=C1COc2ccc(OCC3CCN(CCOc4cccc5ncccc45)C3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-24-16-31-23-7-6-18(13-21(23)26-24)30-15-17-8-10-27(14-17)11-12-29-22-5-1-4-20-19(22)3-2-9-25-20/h1-7,9,13,17H,8,10-12,14-16H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474977

(CHEMBL183901)Show SMILES O=C1COc2ccc(OCC3CCCN(CCOc4cccc5ncccc45)C3)cc2N1 Show InChI InChI=1S/C25H27N3O4/c29-25-17-32-24-9-8-19(14-22(24)27-25)31-16-18-4-3-11-28(15-18)12-13-30-23-7-1-6-21-20(23)5-2-10-26-21/h1-2,5-10,14,18H,3-4,11-13,15-17H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027184

(CHEMBL180700)Show InChI InChI=1S/C21H23N3O4/c25-21-14-28-20-6-5-15(13-18(20)24-21)26-12-10-22-8-2-11-27-19-4-1-3-17-16(19)7-9-23-17/h1,3-7,9,13,22-23H,2,8,10-12,14H2,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474975
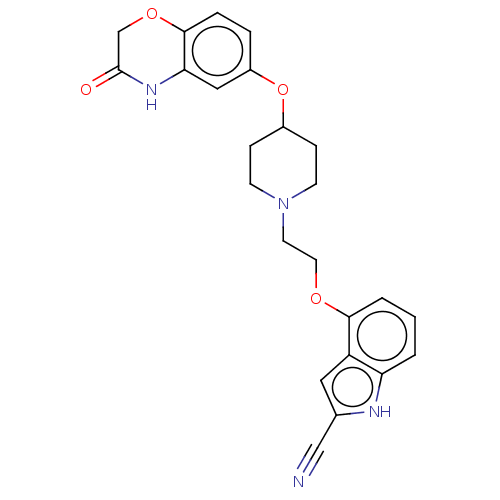
(CHEMBL3706797)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5[nH]c(cc45)C#N)CC3)cc2N1 Show InChI InChI=1S/C24H24N4O4/c25-14-16-12-19-20(26-16)2-1-3-22(19)30-11-10-28-8-6-17(7-9-28)32-18-4-5-23-21(13-18)27-24(29)15-31-23/h1-5,12-13,17,26H,6-11,15H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474980

(CHEMBL180405)Show SMILES CC1(C)OC2C=CC=CC2=C1OCCN1CCC(CC1)Oc1ccc2OCC(=O)Nc2c1 |c:5,7,10| Show InChI InChI=1S/C25H30N2O5/c1-25(2)24(19-5-3-4-6-21(19)32-25)29-14-13-27-11-9-17(10-12-27)31-18-7-8-22-20(15-18)26-23(28)16-30-22/h3-8,15,17,21H,9-14,16H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474979

(CHEMBL180445)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5ccccc45)CC3)cc2N1 Show InChI InChI=1S/C25H26N2O4/c28-25-17-30-24-9-8-20(16-22(24)26-25)31-19-10-12-27(13-11-19)14-15-29-23-7-3-5-18-4-1-2-6-21(18)23/h1-9,16,19H,10-15,17H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085043
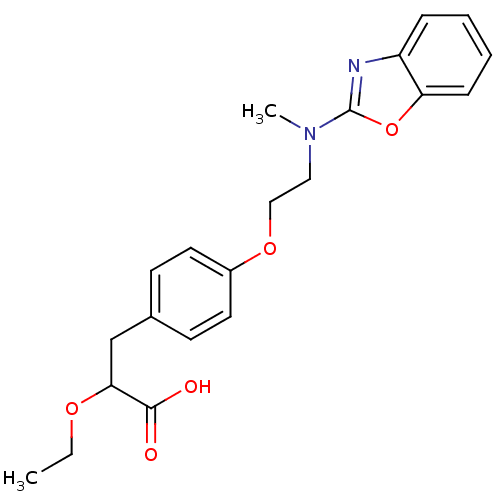
(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show SMILES CCOC(Cc1ccc(OCCN(C)c2nc3ccccc3o2)cc1)C(O)=O Show InChI InChI=1S/C21H24N2O5/c1-3-26-19(20(24)25)14-15-8-10-16(11-9-15)27-13-12-23(2)21-22-17-6-4-5-7-18(17)28-21/h4-11,19H,3,12-14H2,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085043

(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show SMILES CCOC(Cc1ccc(OCCN(C)c2nc3ccccc3o2)cc1)C(O)=O Show InChI InChI=1S/C21H24N2O5/c1-3-26-19(20(24)25)14-15-8-10-16(11-9-15)27-13-12-23(2)21-22-17-6-4-5-7-18(17)28-21/h4-11,19H,3,12-14H2,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2127-2130 (1996)
Article DOI: 10.1016/0960-894X(96)00382-4
BindingDB Entry DOI: 10.7270/Q2J1034Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474981
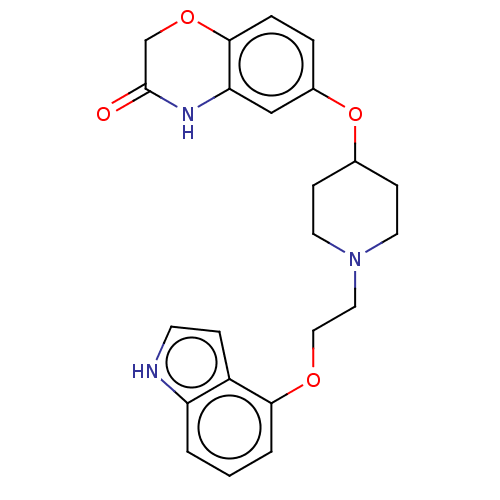
(CHEMBL2113021)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5[nH]ccc45)CC3)cc2N1 Show InChI InChI=1S/C23H25N3O4/c27-23-15-29-22-5-4-17(14-20(22)25-23)30-16-7-10-26(11-8-16)12-13-28-21-3-1-2-19-18(21)6-9-24-19/h1-6,9,14,16,24H,7-8,10-13,15H2,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474981

(CHEMBL2113021)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5[nH]ccc45)CC3)cc2N1 Show InChI InChI=1S/C23H25N3O4/c27-23-15-29-22-5-4-17(14-20(22)25-23)30-16-7-10-26(11-8-16)12-13-28-21-3-1-2-19-18(21)6-9-24-19/h1-6,9,14,16,24H,7-8,10-13,15H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474976

(CHEMBL180557)Show SMILES O=C1COc2ccc(CC3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C25H27N3O3/c29-25-17-31-24-7-6-19(16-22(24)27-25)15-18-8-11-28(12-9-18)13-14-30-23-5-1-4-21-20(23)3-2-10-26-21/h1-7,10,16,18H,8-9,11-15,17H2,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50412114

(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474982

(CHEMBL181186)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5cccnc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-23-16-30-21-7-6-19(15-20(21)26-23)31-18-8-11-27(12-9-18)13-14-29-22-5-1-3-17-4-2-10-25-24(17)22/h1-7,10,15,18H,8-9,11-14,16H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474978
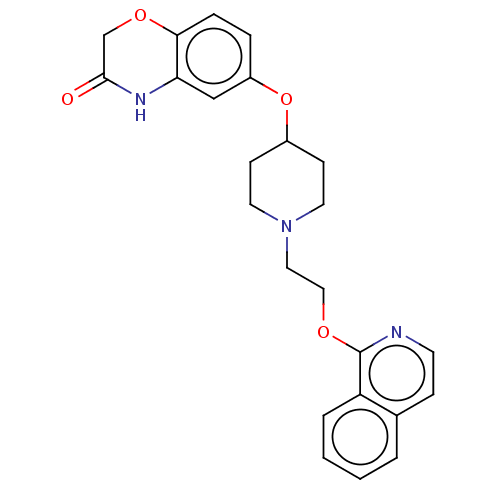
(CHEMBL183551)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4nccc5ccccc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-23-16-30-22-6-5-19(15-21(22)26-23)31-18-8-11-27(12-9-18)13-14-29-24-20-4-2-1-3-17(20)7-10-25-24/h1-7,10,15,18H,8-9,11-14,16H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474975

(CHEMBL3706797)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5[nH]c(cc45)C#N)CC3)cc2N1 Show InChI InChI=1S/C24H24N4O4/c25-14-16-12-19-20(26-16)2-1-3-22(19)30-11-10-28-8-6-17(7-9-28)32-18-4-5-23-21(13-18)27-24(29)15-31-23/h1-5,12-13,17,26H,6-11,15H2,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474985

(CHEMBL180182)Show SMILES O=C1COc2ccc(CN3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C24H26N4O3/c29-24-17-31-23-7-6-18(15-21(23)26-24)16-28-11-9-27(10-12-28)13-14-30-22-5-1-4-20-19(22)3-2-8-25-20/h1-8,15H,9-14,16-17H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50474974

(CHEMBL183332)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-24-16-30-23-7-6-18(15-21(23)26-24)31-17-8-11-27(12-9-17)13-14-29-22-5-1-4-20-19(22)3-2-10-25-20/h1-7,10,15,17H,8-9,11-14,16H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287732

(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show InChI InChI=1S/C20H22N2O5/c1-22(20-21-16-5-3-4-6-17(16)27-20)11-12-26-15-9-7-14(8-10-15)13-18(25-2)19(23)24/h3-10,18H,11-13H2,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50049244
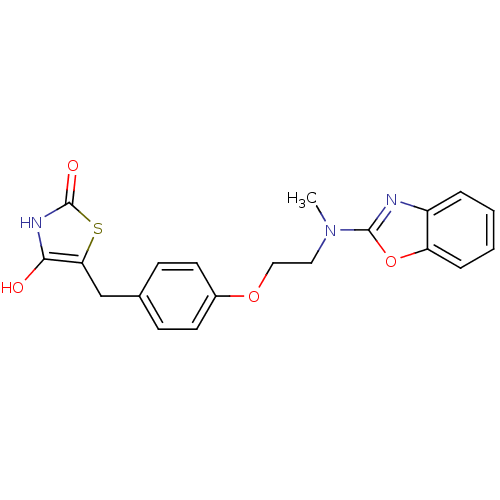
(5-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...)Show SMILES CN(CCOc1ccc(Cc2sc(=O)[nH]c2O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C20H19N3O4S/c1-23(19-21-15-4-2-3-5-16(15)27-19)10-11-26-14-8-6-13(7-9-14)12-17-18(24)22-20(25)28-17/h2-9,24H,10-12H2,1H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50049244

(5-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...)Show SMILES CN(CCOc1ccc(Cc2sc(=O)[nH]c2O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C20H19N3O4S/c1-23(19-21-15-4-2-3-5-16(15)27-19)10-11-26-14-8-6-13(7-9-14)12-17-18(24)22-20(25)28-17/h2-9,24H,10-12H2,1H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2127-2130 (1996)
Article DOI: 10.1016/0960-894X(96)00382-4
BindingDB Entry DOI: 10.7270/Q2J1034Z |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474980

(CHEMBL180405)Show SMILES CC1(C)OC2C=CC=CC2=C1OCCN1CCC(CC1)Oc1ccc2OCC(=O)Nc2c1 |c:5,7,10| Show InChI InChI=1S/C25H30N2O5/c1-25(2)24(19-5-3-4-6-21(19)32-25)29-14-13-27-11-9-17(10-12-27)31-18-7-8-22-20(15-18)26-23(28)16-30-22/h3-8,15,17,21H,9-14,16H2,1-2H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287733

(2-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...)Show SMILES CN(CCOc1ccc(CC(CCCc2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C28H30N2O4/c1-30(28-29-25-12-5-6-13-26(25)34-28)18-19-33-24-16-14-22(15-17-24)20-23(27(31)32)11-7-10-21-8-3-2-4-9-21/h2-6,8-9,12-17,23H,7,10-11,18-20H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2127-2130 (1996)
Article DOI: 10.1016/0960-894X(96)00382-4
BindingDB Entry DOI: 10.7270/Q2J1034Z |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474974

(CHEMBL183332)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-24-16-30-23-7-6-18(15-21(23)26-24)31-17-8-11-27(12-9-17)13-14-29-22-5-1-4-20-19(22)3-2-10-25-20/h1-7,10,15,17H,8-9,11-14,16H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474985

(CHEMBL180182)Show SMILES O=C1COc2ccc(CN3CCN(CCOc4cccc5ncccc45)CC3)cc2N1 Show InChI InChI=1S/C24H26N4O3/c29-24-17-31-23-7-6-18(15-21(23)26-24)16-28-11-9-27(10-12-28)13-14-30-22-5-1-4-20-19(22)3-2-8-25-20/h1-8,15H,9-14,16-17H2,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287727

(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show InChI InChI=1S/C19H19ClN2O4/c1-22(19-21-16-4-2-3-5-17(16)26-19)10-11-25-14-8-6-13(7-9-14)12-15(20)18(23)24/h2-9,15H,10-12H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287730
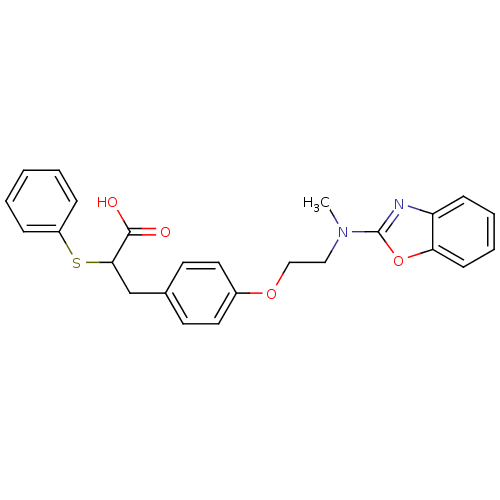
(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show SMILES CN(CCOc1ccc(CC(Sc2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C25H24N2O4S/c1-27(25-26-21-9-5-6-10-22(21)31-25)15-16-30-19-13-11-18(12-14-19)17-23(24(28)29)32-20-7-3-2-4-8-20/h2-14,23H,15-17H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474982

(CHEMBL181186)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5cccnc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-23-16-30-21-7-6-19(15-20(21)26-23)31-18-8-11-27(12-9-18)13-14-29-22-5-1-3-17-4-2-10-25-24(17)22/h1-7,10,15,18H,8-9,11-14,16H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474978

(CHEMBL183551)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4nccc5ccccc45)CC3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-23-16-30-22-6-5-19(15-21(22)26-23)31-18-8-11-27(12-9-18)13-14-29-24-20-4-2-1-3-17(20)7-10-25-24/h1-7,10,15,18H,8-9,11-14,16H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474983

(CHEMBL183034)Show SMILES O=C1COc2ccc(OCC3CCN(CCOc4cccc5ncccc45)C3)cc2N1 Show InChI InChI=1S/C24H25N3O4/c28-24-16-31-23-7-6-18(13-21(23)26-24)30-15-17-8-10-27(14-17)11-12-29-22-5-1-4-20-19(22)3-2-9-25-20/h1-7,9,13,17H,8,10-12,14-16H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474977

(CHEMBL183901)Show SMILES O=C1COc2ccc(OCC3CCCN(CCOc4cccc5ncccc45)C3)cc2N1 Show InChI InChI=1S/C25H27N3O4/c29-25-17-32-24-9-8-19(14-22(24)27-25)31-16-18-4-3-11-28(15-18)12-13-30-23-7-1-6-21-20(23)5-2-10-26-21/h1-2,5-10,14,18H,3-4,11-13,15-17H2,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50027184

(CHEMBL180700)Show InChI InChI=1S/C21H23N3O4/c25-21-14-28-20-6-5-15(13-18(20)24-21)26-12-10-22-8-2-11-27-19-4-1-3-17-16(19)7-9-23-17/h1,3-7,9,13,22-23H,2,8,10-12,14H2,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50474979

(CHEMBL180445)Show SMILES O=C1COc2ccc(OC3CCN(CCOc4cccc5ccccc45)CC3)cc2N1 Show InChI InChI=1S/C25H26N2O4/c28-25-17-30-24-9-8-20(16-22(24)26-25)31-19-10-12-27(13-11-19)14-15-29-23-7-3-5-18-4-1-2-6-21(18)23/h1-9,16,19H,10-15,17H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean negative logarithim of binding affinity was measured for the rat serotonin transporter; n>/=3 |
Bioorg Med Chem Lett 15: 737-41 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.030
BindingDB Entry DOI: 10.7270/Q2RR220M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data