

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420297 (acs.jmedchem.1c00409_ST.30 | med.21724, Compound 6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica | Assay Description Recombinant SARS-CoV-2 Mpro was expressed and purified from Escherichia coli (E. coli) (18, 25). A fluorescently labeled substrate, MCA-AVLQ SGFR-Lys... | Science 368: 1331-1335 (2020) Article DOI: 10.1126/science.abb4489 BindingDB Entry DOI: 10.7270/Q27W6FK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420296 (Advanced SARS-CoV-2 Inhibitor 11a | MPI10 | acs.jm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica | Assay Description Recombinant SARS-CoV-2 Mpro was expressed and purified from Escherichia coli (E. coli) (18, 25). A fluorescently labeled substrate, MCA-AVLQ SGFR-Lys... | Science 368: 1331-1335 (2020) Article DOI: 10.1126/science.abb4489 BindingDB Entry DOI: 10.7270/Q27W6FK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
ShanghaiTech University | Assay Description Recombinant SARS-CoV-2 Mpro with native N and C termini was expressed in Escherichia coli, and subsequently purified (Extended Data Fig. 1a, b). The ... | Nature 582: 289-293 (2020) Article DOI: 10.1038/s41586-020-2223-y BindingDB Entry DOI: 10.7270/Q25B04WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50166940 (NP-031112 | NP-12 | Tideglusib | US20230414581, Co...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ShanghaiTech University | Assay Description Recombinant SARS-CoV-2 Mpro with native N and C termini was expressed in Escherichia coli, and subsequently purified (Extended Data Fig. 1a, b). The ... | Nature 582: 289-293 (2020) Article DOI: 10.1038/s41586-020-2223-y BindingDB Entry DOI: 10.7270/Q25B04WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50431275 (CARMOFUR | Carm-ofur | Mifurol | med.21724, Compou...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ShanghaiTech University | Assay Description Recombinant SARS-CoV-2 Mpro with native N and C termini was expressed in Escherichia coli, and subsequently purified (Extended Data Fig. 1a, b). The ... | Nature 582: 289-293 (2020) Article DOI: 10.1038/s41586-020-2223-y BindingDB Entry DOI: 10.7270/Q25B04WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50058655 (1,1',1'',1'''-[disulfanediylbis(carbonothioylnitri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ShanghaiTech University | Assay Description Recombinant SARS-CoV-2 Mpro with native N and C termini was expressed in Escherichia coli, and subsequently purified (Extended Data Fig. 1a, b). The ... | Nature 582: 289-293 (2020) Article DOI: 10.1038/s41586-020-2223-y BindingDB Entry DOI: 10.7270/Q25B04WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM178090 (5,8-dihydroxy-2-(1-hydroxy-4-methylpent-3-enyl)nap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ShanghaiTech University | Assay Description Recombinant SARS-CoV-2 Mpro with native N and C termini was expressed in Escherichia coli, and subsequently purified (Extended Data Fig. 1a, b). The ... | Nature 582: 289-293 (2020) Article DOI: 10.1038/s41586-020-2223-y BindingDB Entry DOI: 10.7270/Q25B04WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497232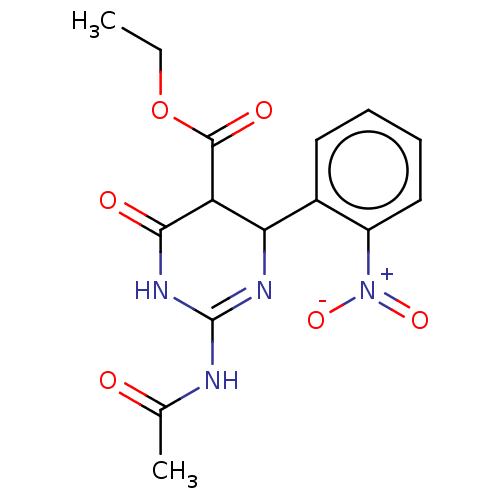 (CHEMBL3290246) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50426071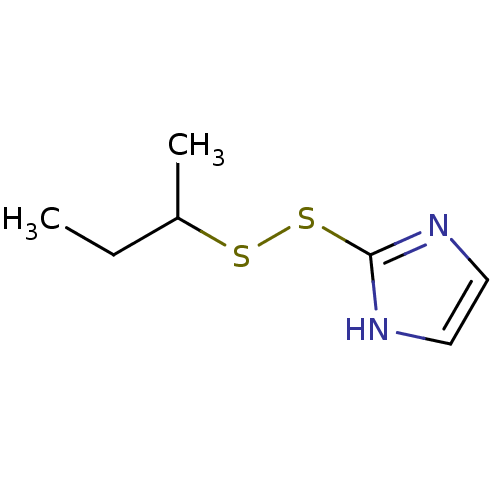 (CHEMBL406050 | PX-12 | US9018255, PX-12 | med.2172...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ShanghaiTech University | Assay Description Recombinant SARS-CoV-2 Mpro with native N and C termini was expressed in Escherichia coli, and subsequently purified (Extended Data Fig. 1a, b). The ... | Nature 582: 289-293 (2020) Article DOI: 10.1038/s41586-020-2223-y BindingDB Entry DOI: 10.7270/Q25B04WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497231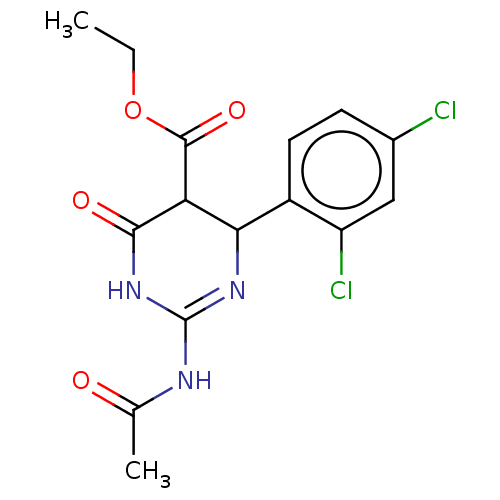 (CHEMBL3290243) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497249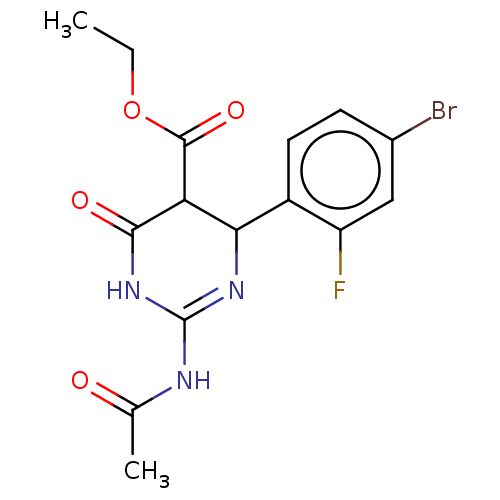 (CHEMBL3290244) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497240 (CHEMBL3286435) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497242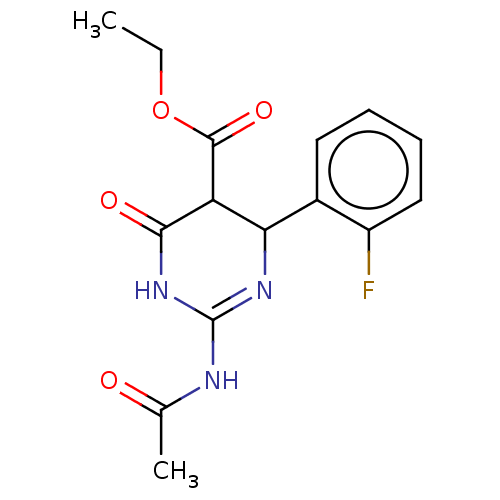 (CHEMBL3290254) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497230 (CHEMBL3290242) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497223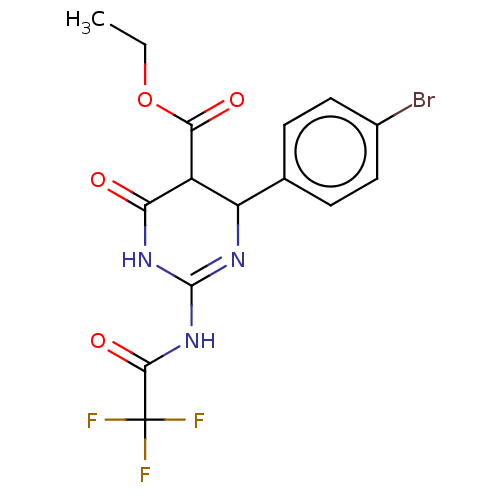 (CHEMBL3290257) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497221 (CHEMBL3290252) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497237 (CHEMBL3290231) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497248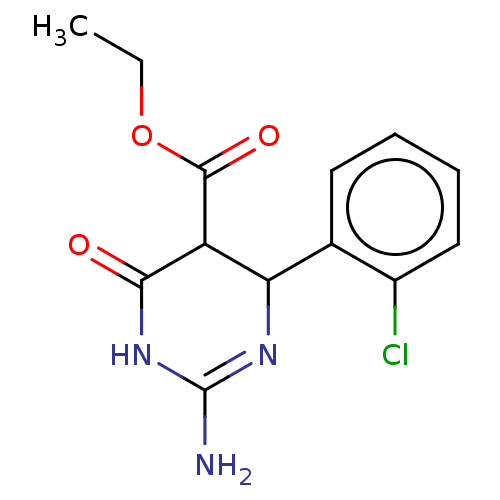 (CHEMBL3290229) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497219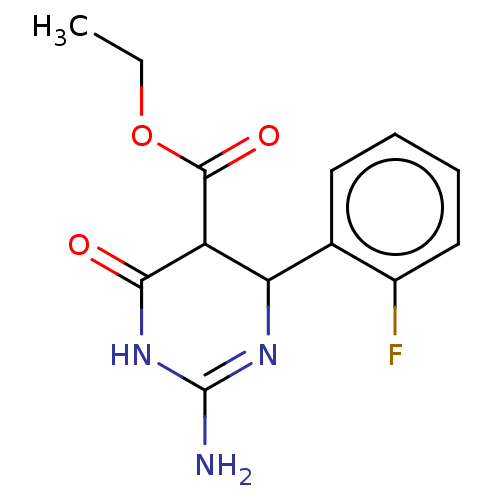 (CHEMBL3290239) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497234 (CHEMBL3290251) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497241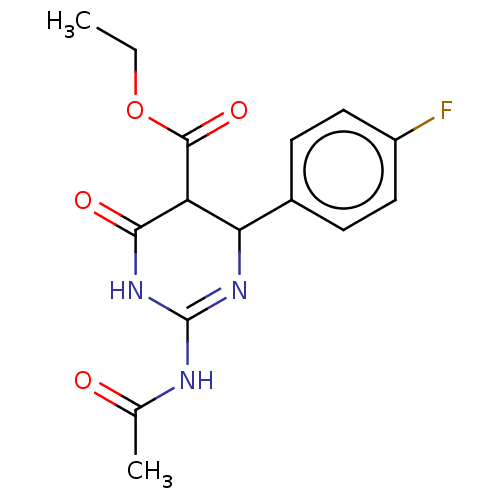 (CHEMBL3290249) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497236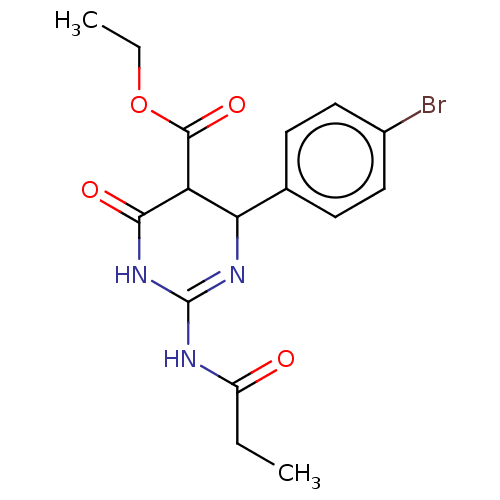 (CHEMBL3290256) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497222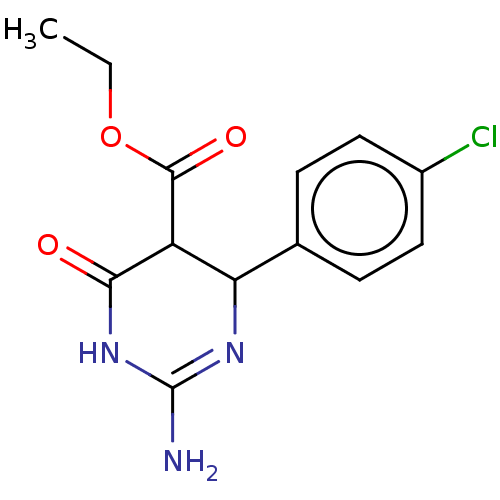 (CHEMBL3290236) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497243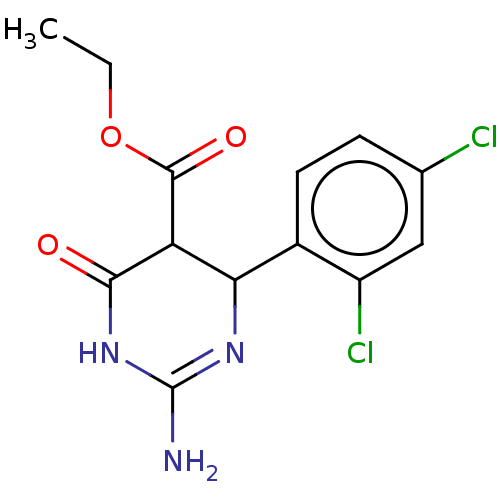 (CHEMBL3290227) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497245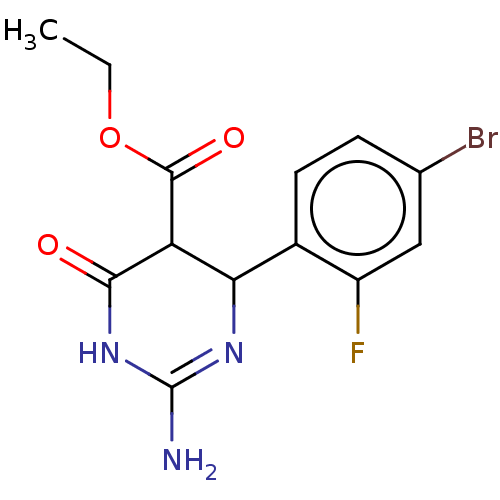 (CHEMBL3290228) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497238 (CHEMBL3290237) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM419120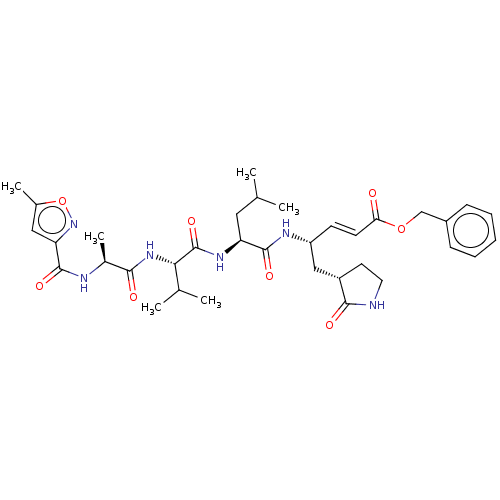 (acs.jmedchem.1c00409_ST.487 | cmdc.202100576, 8d(N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ShanghaiTech University | Assay Description Recombinant SARS-CoV-2 Mpro with native N and C termini was expressed in Escherichia coli, and subsequently purified (Extended Data Fig. 1a, b). The ... | Nature 582: 289-293 (2020) Article DOI: 10.1038/s41586-020-2223-y BindingDB Entry DOI: 10.7270/Q25B04WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497217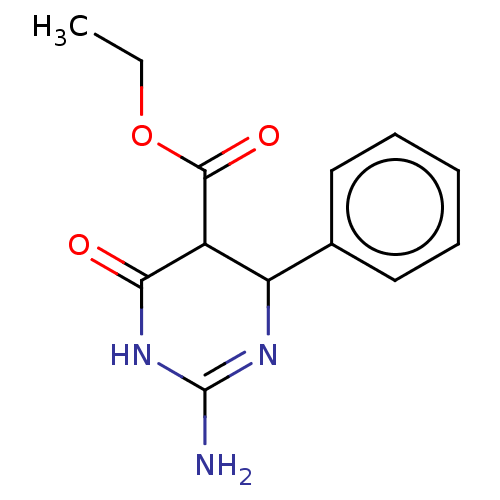 (CHEMBL3290225) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497246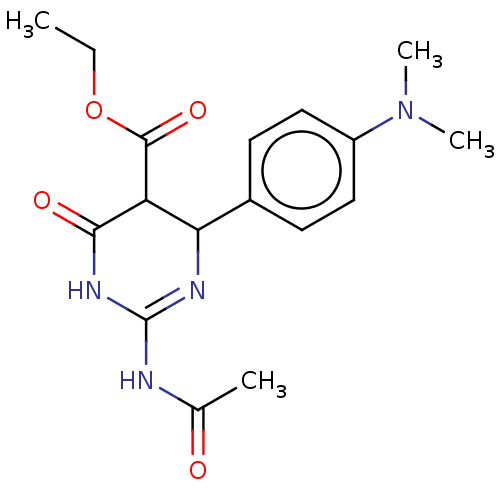 (CHEMBL3290255) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497226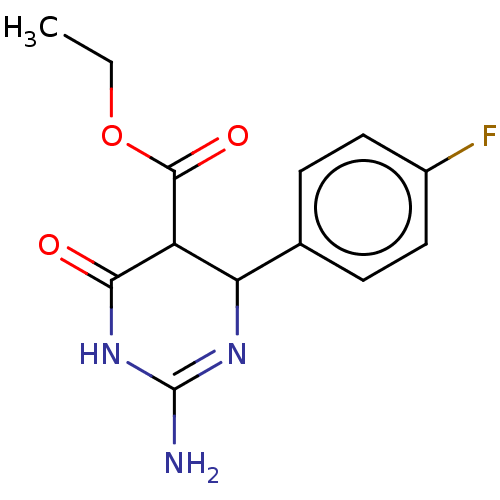 (CHEMBL3290234) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497247 (CHEMBL3290247) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497220 (CHEMBL3290245) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497244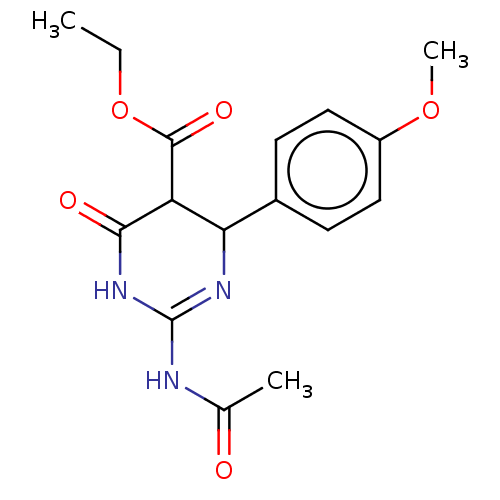 (CHEMBL3290250) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497225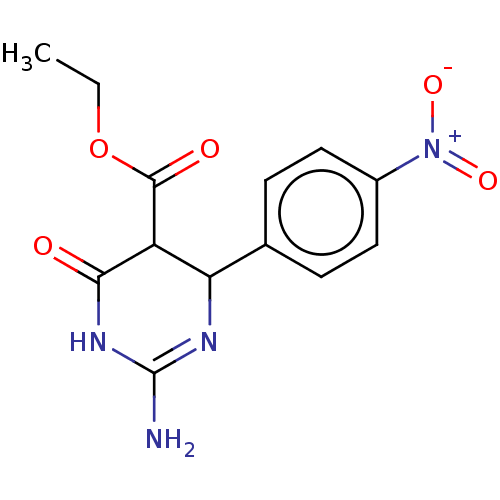 (CHEMBL3290232) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497216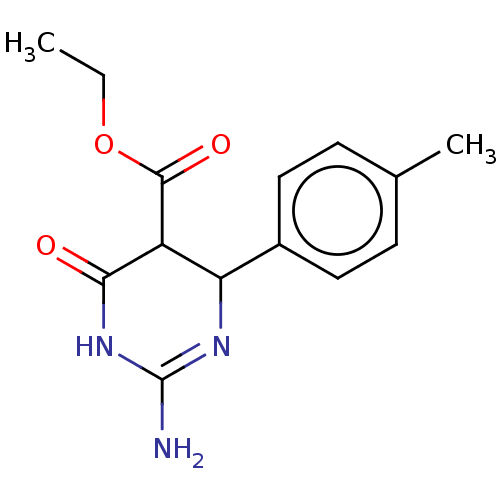 (CHEMBL3290230) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497218 (CHEMBL3290233) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497224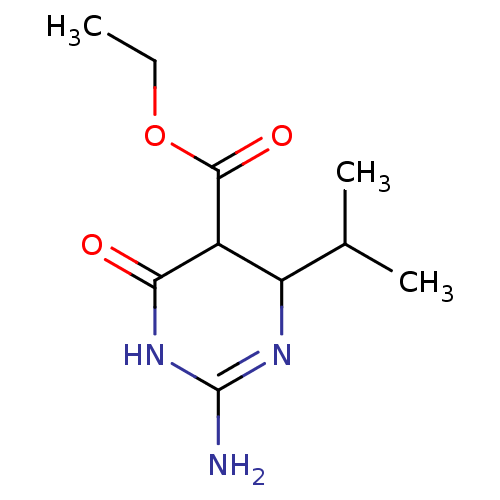 (CHEMBL3290226) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497227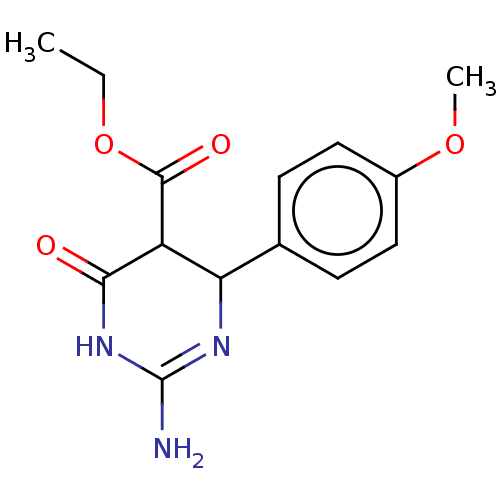 (CHEMBL3290235) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497228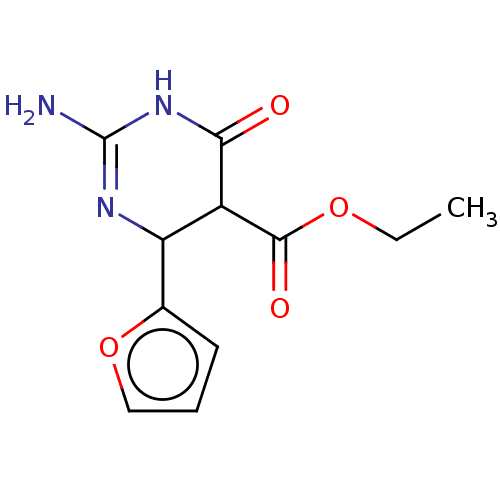 (CHEMBL3290238) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497229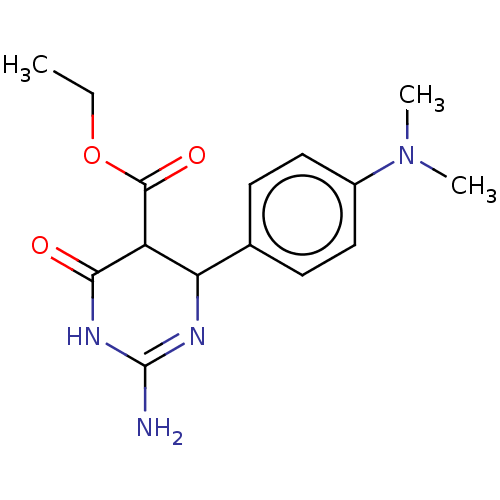 (CHEMBL3290240) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497233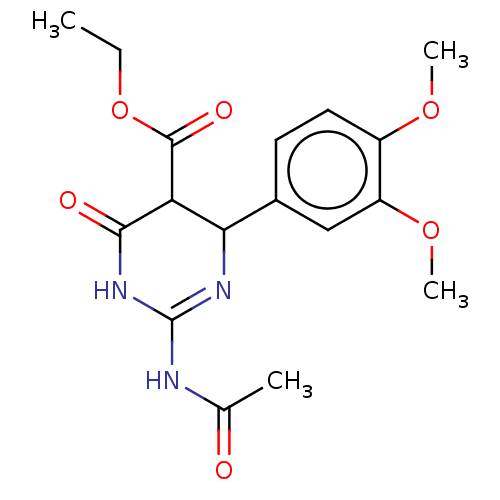 (CHEMBL3290248) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497235 (CHEMBL3290253) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50497239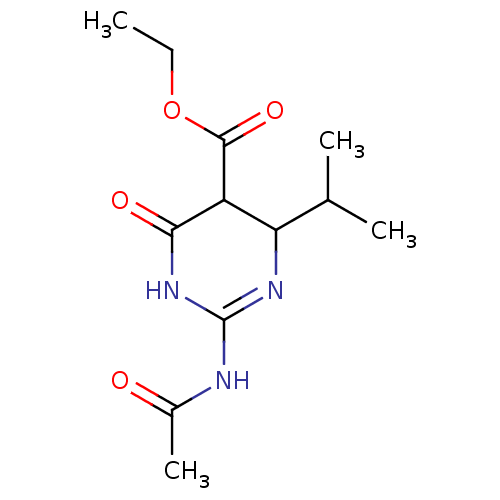 (CHEMBL3290241) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A H1N1 virus neuraminidase after 1 hr by spectrofluorimetry using 2-(4-meythylumbelliferyl)-alpha-D-acetylneuramic acid as su... | Eur J Med Chem 83: 466-73 (2014) Article DOI: 10.1016/j.ejmech.2014.06.059 BindingDB Entry DOI: 10.7270/Q2251N6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay | Bioorg Med Chem Lett 25: 4158-63 (2015) Article DOI: 10.1016/j.bmcl.2015.08.011 BindingDB Entry DOI: 10.7270/Q2GM8938 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50038210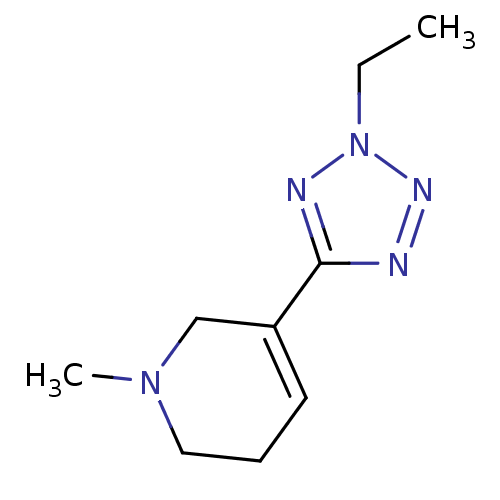 (5-(2-Ethyl-2H-tetrazol-5-yl)-1-methyl-1,2,3,6-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 296 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay | Bioorg Med Chem Lett 25: 4158-63 (2015) Article DOI: 10.1016/j.bmcl.2015.08.011 BindingDB Entry DOI: 10.7270/Q2GM8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50061705 ((R)-1-Aza-bicyclo[2.2.2]oct-3-yl-[(Z)-methoxyimino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay | Bioorg Med Chem Lett 25: 4158-63 (2015) Article DOI: 10.1016/j.bmcl.2015.08.011 BindingDB Entry DOI: 10.7270/Q2GM8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50119648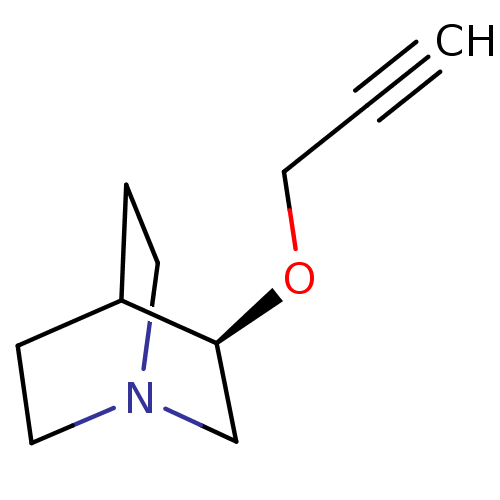 (Talsaclidine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 849 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay | Bioorg Med Chem Lett 25: 4158-63 (2015) Article DOI: 10.1016/j.bmcl.2015.08.011 BindingDB Entry DOI: 10.7270/Q2GM8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50003359 (5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 121 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay | Bioorg Med Chem Lett 25: 4158-63 (2015) Article DOI: 10.1016/j.bmcl.2015.08.011 BindingDB Entry DOI: 10.7270/Q2GM8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 205 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay | Bioorg Med Chem Lett 25: 4158-63 (2015) Article DOI: 10.1016/j.bmcl.2015.08.011 BindingDB Entry DOI: 10.7270/Q2GM8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 114 total ) | Next | Last >> |