| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Muscarinic acetylcholine receptor M2 |
|---|
| Ligand | BDBM50038210 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1517984 (CHEMBL3618841) |
|---|
| EC50 | 296±n/a nM |
|---|
| Citation |  Liu, B; Croy, CH; Hitchcock, SA; Allen, JR; Rao, Z; Evans, D; Bures, MG; McKinzie, DL; Watt, ML; Stuart Gregory, G; Hansen, MM; Hoogestraat, PJ; Jamison, JA; Okha-Mokube, FM; Stratford, RE; Turner, W; Bymaster, F; Felder, CC Design and synthesis of N-[6-(Substituted Aminoethylideneamino)-2-Hydroxyindan-1-yl]arylamides as selective and potent muscarinic M1 agonists. Bioorg Med Chem Lett25:4158-63 (2015) [PubMed] Article Liu, B; Croy, CH; Hitchcock, SA; Allen, JR; Rao, Z; Evans, D; Bures, MG; McKinzie, DL; Watt, ML; Stuart Gregory, G; Hansen, MM; Hoogestraat, PJ; Jamison, JA; Okha-Mokube, FM; Stratford, RE; Turner, W; Bymaster, F; Felder, CC Design and synthesis of N-[6-(Substituted Aminoethylideneamino)-2-Hydroxyindan-1-yl]arylamides as selective and potent muscarinic M1 agonists. Bioorg Med Chem Lett25:4158-63 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Muscarinic acetylcholine receptor M2 |
|---|
| Name: | Muscarinic acetylcholine receptor M2 |
|---|
| Synonyms: | ACM2_HUMAN | CHRM2 | Cholinergic, muscarinic M2 | Muscarinic acetylcholine receptor M2 and M4 | Muscarinic acetylcholine receptor M2 and M5 | RecName: Full=Muscarinic acetylcholine receptor M2 |
|---|
| Type: | GPCR |
|---|
| Mol. Mass.: | 51730.61 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P08172 |
|---|
| Residue: | 466 |
|---|
| Sequence: | MNNSTNSSNNSLALTSPYKTFEVVFIVLVAGSLSLVTIIGNILVMVSIKVNRHLQTVNNY
FLFSLACADLIIGVFSMNLYTLYTVIGYWPLGPVVCDLWLALDYVVSNASVMNLLIISFD
RYFCVTKPLTYPVKRTTKMAGMMIAAAWVLSFILWAPAILFWQFIVGVRTVEDGECYIQF
FSNAAVTFGTAIAAFYLPVIIMTVLYWHISRASKSRIKKDKKEPVANQDPVSPSLVQGRI
VKPNNNNMPSSDDGLEHNKIQNGKAPRDPVTENCVQGEEKESSNDSTSVSAVASNMRDDE
ITQDENTVSTSLGHSKDENSKQTCIRIGTKTPKSDSCTPTNTTVEVVGSSGQNGDEKQNI
VARKIVKMTKQPAKKKPPPSREKKVTRTILAILLAFIITWAPYNVMVLINTFCAPCIPNT
VWTIGYWLCYINSTINPACYALCNATFKKTFKHLLMCHYKNIGATR
|
|
|
|---|
| BDBM50038210 |
|---|
| n/a |
|---|
| Name | BDBM50038210 |
|---|
| Synonyms: | 5-(2-Ethyl-2H-tetrazol-5-yl)-1-methyl-1,2,3,6-tetrahydro-pyridine | Alvameline | CHEMBL131428 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C9H15N5 |
|---|
| Mol. Mass. | 193.2489 |
|---|
| SMILES | CCn1nnc(n1)C1=CCCN(C)C1 |t:8| |
|---|
| Structure | 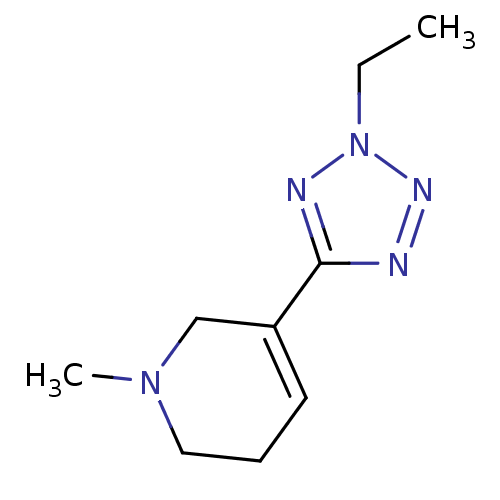
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liu, B; Croy, CH; Hitchcock, SA; Allen, JR; Rao, Z; Evans, D; Bures, MG; McKinzie, DL; Watt, ML; Stuart Gregory, G; Hansen, MM; Hoogestraat, PJ; Jamison, JA; Okha-Mokube, FM; Stratford, RE; Turner, W; Bymaster, F; Felder, CC Design and synthesis of N-[6-(Substituted Aminoethylideneamino)-2-Hydroxyindan-1-yl]arylamides as selective and potent muscarinic M1 agonists. Bioorg Med Chem Lett25:4158-63 (2015) [PubMed] Article
Liu, B; Croy, CH; Hitchcock, SA; Allen, JR; Rao, Z; Evans, D; Bures, MG; McKinzie, DL; Watt, ML; Stuart Gregory, G; Hansen, MM; Hoogestraat, PJ; Jamison, JA; Okha-Mokube, FM; Stratford, RE; Turner, W; Bymaster, F; Felder, CC Design and synthesis of N-[6-(Substituted Aminoethylideneamino)-2-Hydroxyindan-1-yl]arylamides as selective and potent muscarinic M1 agonists. Bioorg Med Chem Lett25:4158-63 (2015) [PubMed] Article