 Found 116 hits with Last Name = 'watt' and Initial = 'ml'
Found 116 hits with Last Name = 'watt' and Initial = 'ml' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406127
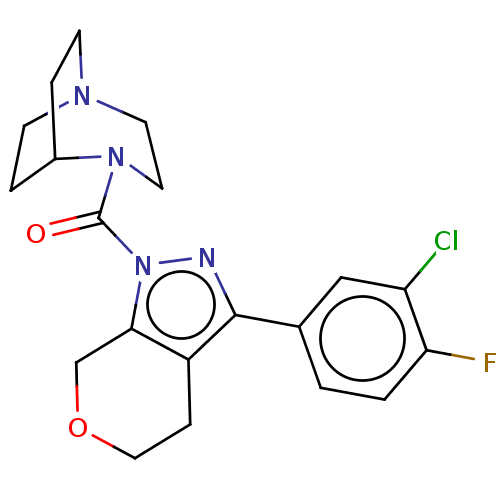
(CHEMBL5290844)Show SMILES Cc1cc(C)c(CCCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C22H28FO5P/c1-14-9-15(2)19(20(10-14)17-6-7-21(23)16(3)11-17)5-4-8-29(27,28)13-18(24)12-22(25)26/h6-7,9-11,27-29H,4-5,8,12-13H2,1-3H3,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406126

(CHEMBL5291288)Show SMILES CC(C)c1c(CCP(O)(O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 Show InChI InChI=1S/C23H27FNO5P/c1-15(2)23-19-5-3-4-6-20(19)25(17-9-7-16(24)8-10-17)21(23)11-12-31(29,30)14-18(26)13-22(27)28/h3-10,15,29-31H,11-14H2,1-2H3,(H,27,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406123
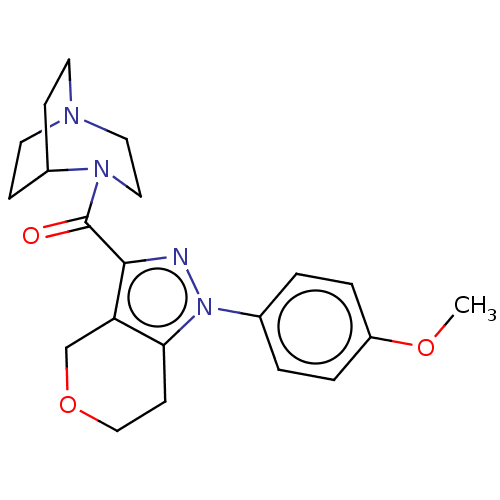
(CHEMBL5277419)Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1CCP(O)(O)CC(=O)CC(O)=O Show InChI InChI=1S/C23H30FO5P/c1-14(2)20-9-15(3)10-21(17-5-6-22(24)16(4)11-17)19(20)7-8-30(28,29)13-18(25)12-23(26)27/h5-6,9-11,14,28-30H,7-8,12-13H2,1-4H3,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50593123
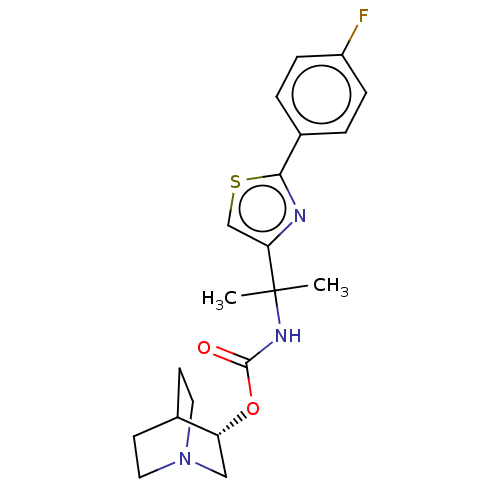
(GENZ-682452 | GZ-402671 | GZ/SAR402671 | GZ402671 ...)Show SMILES CC(C)(NC(=O)O[C@@H]1CN2CCC1CC2)c1csc(n1)-c1ccc(F)cc1 |r,wU:7.6,(-6.59,.2,;-5.85,-1.18,;-5.38,.09,;-4.52,-1.95,;-3.19,-1.18,;-3.19,.36,;-1.85,-1.95,;-.52,-1.18,;.81,-1.95,;2.15,-1.18,;2.15,.36,;.81,1.13,;-.52,.36,;.5,.07,;1.39,-.44,;-7.1,-2.08,;-7.1,-3.62,;-8.57,-4.1,;-9.47,-2.85,;-8.57,-1.6,;-11.01,-2.85,;-11.78,-1.52,;-13.32,-1.52,;-14.09,-2.85,;-15.63,-2.85,;-13.32,-4.18,;-11.78,-4.18,)| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against bovine cathepsin D |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Mus musculus) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Mus musculus) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406132

(CHEMBL5273314)Show SMILES CC(C)n1c(C=CP(O)(=O)CC(=O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)25-20-6-4-3-5-19(20)23(16-7-9-17(24)10-8-16)21(25)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406137

(CHEMBL5286066)Show InChI InChI=1S/C21H27N3O/c1-18-7-5-6-10-20(18)21(25)22-11-12-23-13-15-24(16-14-23)17-19-8-3-2-4-9-19/h2-10H,11-17H2,1H3,(H,22,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406133

(CHEMBL5288633)Show SMILES COP(=O)(C[C@@H](O)CC(O)=O)NCc1c(C)cc(C)cc1-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H27FNO5P/c1-13-7-14(2)19(18(8-13)16-5-6-20(22)15(3)9-16)11-23-29(27,28-4)12-17(24)10-21(25)26/h5-9,17,24H,10-12H2,1-4H3,(H,23,27)(H,25,26)/t17-,29?/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406136

(CHEMBL5278676)Show SMILES CCc1c(C=CP(O)(=O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 |w:5.5| Show InChI InChI=1S/C22H21FNO5P/c1-2-18-19-5-3-4-6-20(19)24(16-9-7-15(23)8-10-16)21(18)11-12-30(28,29)14-17(25)13-22(26)27/h3-12H,2,13-14H2,1H3,(H,26,27)(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against aspartic proteinases pepsin from porcine |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406138
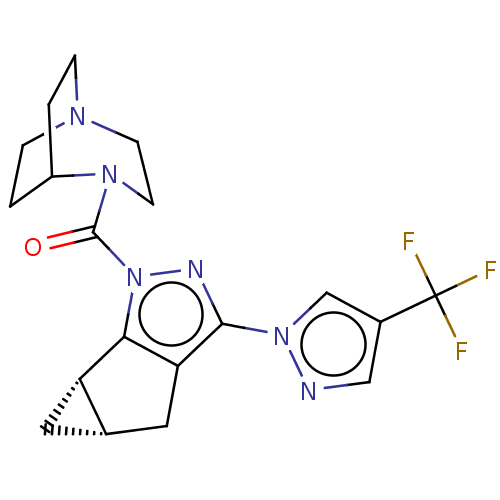
(CHEMBL5286071)Show SMILES [O-][N+](=O)c1ccccc1NC(=O)CCN1CCN2Cc3[nH]c4ccccc4c3CC2C1 Show InChI InChI=1S/C23H25N5O3/c29-23(25-20-7-3-4-8-22(20)28(30)31)9-10-26-11-12-27-15-21-18(13-16(27)14-26)17-5-1-2-6-19(17)24-21/h1-8,16,24H,9-15H2,(H,25,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406131
(CHEMBL5278405)Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1C=CP(O)(=O)CC(=O)CC(O)=O |w:19.21| Show InChI InChI=1S/C23H26FO5P/c1-14(2)20-9-15(3)10-21(17-5-6-22(24)16(4)11-17)19(20)7-8-30(28,29)13-18(25)12-23(26)27/h5-11,14H,12-13H2,1-4H3,(H,26,27)(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406135

(CHEMBL5278640)Show SMILES CC(C)c1c(C=CP(O)(=O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)23-19-5-3-4-6-20(19)25(17-9-7-16(24)8-10-17)21(23)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50593123

(GENZ-682452 | GZ-402671 | GZ/SAR402671 | GZ402671 ...)Show SMILES CC(C)(NC(=O)O[C@@H]1CN2CCC1CC2)c1csc(n1)-c1ccc(F)cc1 |r,wU:7.6,(-6.59,.2,;-5.85,-1.18,;-5.38,.09,;-4.52,-1.95,;-3.19,-1.18,;-3.19,.36,;-1.85,-1.95,;-.52,-1.18,;.81,-1.95,;2.15,-1.18,;2.15,.36,;.81,1.13,;-.52,.36,;.5,.07,;1.39,-.44,;-7.1,-2.08,;-7.1,-3.62,;-8.57,-4.1,;-9.47,-2.85,;-8.57,-1.6,;-11.01,-2.85,;-11.78,-1.52,;-13.32,-1.52,;-14.09,-2.85,;-15.63,-2.85,;-13.32,-4.18,;-11.78,-4.18,)| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406119

(CHEMBL5273405)Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1COP(O)(=O)C[C@@H](O)CC(O)=O Show InChI InChI=1S/C22H28FO6P/c1-13(2)18-7-14(3)8-19(16-5-6-21(23)15(4)9-16)20(18)11-29-30(27,28)12-17(24)10-22(25)26/h5-9,13,17,24H,10-12H2,1-4H3,(H,25,26)(H,27,28)/t17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406128

(CHEMBL5270537)Show SMILES OC(=O)CC(=O)CP(O)(=O)CCC1=C(c2ccccc2C11CCCC1)c1ccc(F)cc1 |t:12| Show InChI InChI=1S/C25H26FO5P/c26-18-9-7-17(8-10-18)24-20-5-1-2-6-21(20)25(12-3-4-13-25)22(24)11-14-32(30,31)16-19(27)15-23(28)29/h1-2,5-10H,3-4,11-16H2,(H,28,29)(H,30,31) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406131

(CHEMBL5278405)Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1C=CP(O)(=O)CC(=O)CC(O)=O |w:19.21| Show InChI InChI=1S/C23H26FO5P/c1-14(2)20-9-15(3)10-21(17-5-6-22(24)16(4)11-17)19(20)7-8-30(28,29)13-18(25)12-23(26)27/h5-11,14H,12-13H2,1-4H3,(H,26,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 338 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
ETA receptor antagonist activity was measured by inhibition of ET-1 induced vasoconstriction in isolated porcine coronary artery |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406135

(CHEMBL5278640)Show SMILES CC(C)c1c(C=CP(O)(=O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)23-19-5-3-4-6-20(19)25(17-9-7-16(24)8-10-17)21(23)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 585 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406118
(CHEMBL5281181)Show SMILES CC(C)n1c(CCP(O)(O)CC(=O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 Show InChI InChI=1S/C23H27FNO5P/c1-15(2)25-20-6-4-3-5-19(20)23(16-7-9-17(24)10-8-16)21(25)11-12-31(29,30)14-18(26)13-22(27)28/h3-10,15,29-31H,11-14H2,1-2H3,(H,27,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406128

(CHEMBL5270537)Show SMILES OC(=O)CC(=O)CP(O)(=O)CCC1=C(c2ccccc2C11CCCC1)c1ccc(F)cc1 |t:12| Show InChI InChI=1S/C25H26FO5P/c26-18-9-7-17(8-10-18)24-20-5-1-2-6-21(20)25(12-3-4-13-25)22(24)11-14-32(30,31)16-19(27)15-23(28)29/h1-2,5-10H,3-4,11-16H2,(H,28,29)(H,30,31) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406132

(CHEMBL5273314)Show SMILES CC(C)n1c(C=CP(O)(=O)CC(=O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)25-20-6-4-3-5-19(20)23(16-7-9-17(24)10-8-16)21(25)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406137

(CHEMBL5286066)Show InChI InChI=1S/C21H27N3O/c1-18-7-5-6-10-20(18)21(25)22-11-12-23-13-15-24(16-14-23)17-19-8-3-2-4-9-19/h2-10H,11-17H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406138

(CHEMBL5286071)Show SMILES [O-][N+](=O)c1ccccc1NC(=O)CCN1CCN2Cc3[nH]c4ccccc4c3CC2C1 Show InChI InChI=1S/C23H25N5O3/c29-23(25-20-7-3-4-8-22(20)28(30)31)9-10-26-11-12-27-15-21-18(13-16(27)14-26)17-5-1-2-6-19(17)24-21/h1-8,16,24H,9-15H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406133

(CHEMBL5288633)Show SMILES COP(=O)(C[C@@H](O)CC(O)=O)NCc1c(C)cc(C)cc1-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H27FNO5P/c1-13-7-14(2)19(18(8-13)16-5-6-20(22)15(3)9-16)11-23-29(27,28-4)12-17(24)10-21(25)26/h5-9,17,24H,10-12H2,1-4H3,(H,23,27)(H,25,26)/t17-,29?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 4.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406122
(CHEMBL5286114)Show SMILES OC(=O)CC(=O)CP(O)(=O)C=CC1=C(c2ccccc2C11CCCC1)c1ccc(F)cc1 |w:10.9,t:12| Show InChI InChI=1S/C25H24FO5P/c26-18-9-7-17(8-10-18)24-20-5-1-2-6-21(20)25(12-3-4-13-25)22(24)11-14-32(30,31)16-19(27)15-23(28)29/h1-2,5-11,14H,3-4,12-13,15-16H2,(H,28,29)(H,30,31) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406136

(CHEMBL5278676)Show SMILES CCc1c(C=CP(O)(=O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 |w:5.5| Show InChI InChI=1S/C22H21FNO5P/c1-2-18-19-5-3-4-6-20(19)24(16-9-7-15(23)8-10-16)21(18)11-12-30(28,29)14-17(25)13-22(26)27/h3-12H,2,13-14H2,1H3,(H,26,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406117
(CHEMBL5288301)Show SMILES COP(=O)(CCc1c(C)cc(C)cc1-c1ccc(F)c(C)c1)C[C@@H](O)CC(O)=O Show InChI InChI=1S/C22H28FO5P/c1-14-9-15(2)19(20(10-14)17-5-6-21(23)16(3)11-17)7-8-29(27,28-4)13-18(24)12-22(25)26/h5-6,9-11,18,24H,7-8,12-13H2,1-4H3,(H,25,26)/t18-,29?/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was evaluated for the inhibition of microsomal 2,3-oxidosqualene-lanosterol cyclase in Candida albicans microsomes |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406125
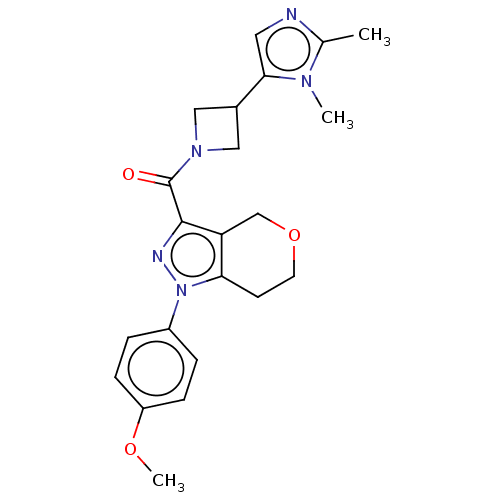
(CHEMBL5291482)Show SMILES OC(=O)CC(=O)CP(O)(=O)C=CC1=C(c2ccccc2C11CC1)c1ccc(F)cc1 |w:10.9,t:12| Show InChI InChI=1S/C23H20FO5P/c24-16-7-5-15(6-8-16)22-18-3-1-2-4-19(18)23(10-11-23)20(22)9-12-30(28,29)14-17(25)13-21(26)27/h1-9,12H,10-11,13-14H2,(H,26,27)(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406124

(CHEMBL5277877)Show SMILES CC(C)n1c(CCP(O)(=O)CC(=O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 Show InChI InChI=1S/C23H25FNO5P/c1-15(2)25-20-6-4-3-5-19(20)23(16-7-9-17(24)10-8-16)21(25)11-12-31(29,30)14-18(26)13-22(27)28/h3-10,15H,11-14H2,1-2H3,(H,27,28)(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406120
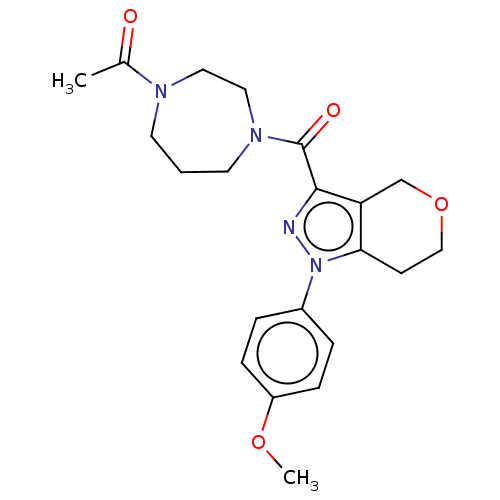
(CHEMBL5267915)Show SMILES Cc1cc(C)c(CP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C20H24FO5P/c1-12-6-13(2)18(11-27(25,26)10-16(22)9-20(23)24)17(7-12)15-4-5-19(21)14(3)8-15/h4-8,25-27H,9-11H2,1-3H3,(H,23,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | >2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50038210
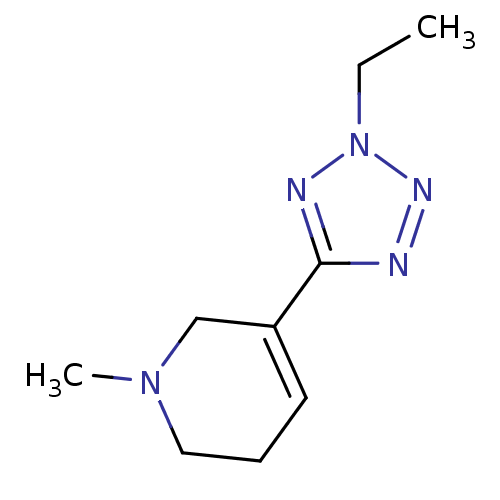
(5-(2-Ethyl-2H-tetrazol-5-yl)-1-methyl-1,2,3,6-tetr...)Show InChI InChI=1S/C9H15N5/c1-3-14-11-9(10-12-14)8-5-4-6-13(2)7-8/h5H,3-4,6-7H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 296 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50061705

((R)-1-Aza-bicyclo[2.2.2]oct-3-yl-[(Z)-methoxyimino...)Show SMILES CO\N=C(/C#N)[C@H]1CN2CCC1CC2 |wU:6.5,(10.77,-7.56,;9.23,-7.56,;8.14,-8.66,;8.14,-10.2,;9.41,-10.96,;10.69,-11.69,;6.8,-10.98,;6.8,-12.53,;5.46,-13.28,;4.14,-12.53,;4.14,-10.98,;5.46,-10.2,;4.95,-11.51,;5.89,-12.1,)| Show InChI InChI=1S/C10H15N3O/c1-14-12-10(6-11)9-7-13-4-2-8(9)3-5-13/h8-9H,2-5,7H2,1H3/b12-10+/t9-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human muscarinic M2 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay |
Bioorg Med Chem Lett 25: 4158-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.011
BindingDB Entry DOI: 10.7270/Q2GM8938 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data