 Found 13311 hits with Last Name = 'ray' and Initial = 's'
Found 13311 hits with Last Name = 'ray' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456924
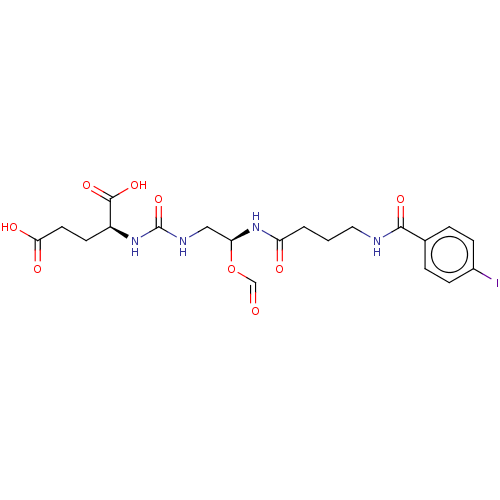
(US10736974, Compound YC-I-27)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(I)cc1)OC=O)C(O)=O |r| Show InChI InChI=1S/C20H25IN4O9/c21-13-5-3-12(4-6-13)18(30)22-9-1-2-15(27)25-16(34-11-26)10-23-20(33)24-14(19(31)32)7-8-17(28)29/h3-6,11,14,16H,1-2,7-10H2,(H,22,30)(H,25,27)(H,28,29)(H,31,32)(H2,23,24,33)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456928
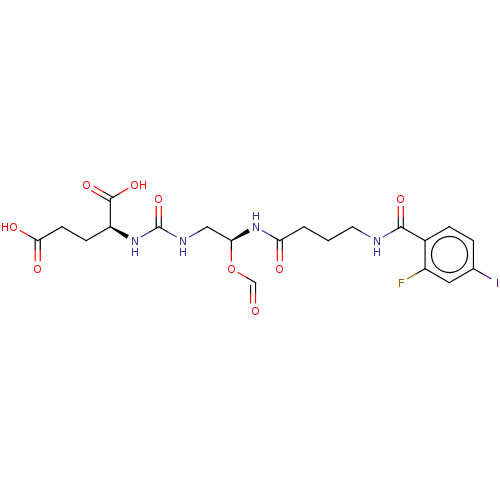
(US10736974, Compound XY-44)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(I)cc1F)OC=O)C(O)=O |r| Show InChI InChI=1S/C20H24FIN4O9/c21-13-8-11(22)3-4-12(13)18(31)23-7-1-2-15(28)26-16(35-10-27)9-24-20(34)25-14(19(32)33)5-6-17(29)30/h3-4,8,10,14,16H,1-2,5-7,9H2,(H,23,31)(H,26,28)(H,29,30)(H,32,33)(H2,24,25,34)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456930

(US10736974, Compound XY-59)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(Br)cc1)OC=O)C(O)=O |r| Show InChI InChI=1S/C20H25BrN4O9/c21-13-5-3-12(4-6-13)18(30)22-9-1-2-15(27)25-16(34-11-26)10-23-20(33)24-14(19(31)32)7-8-17(28)29/h3-6,11,14,16H,1-2,7-10H2,(H,22,30)(H,25,27)(H,28,29)(H,31,32)(H2,23,24,33)/t14-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456923
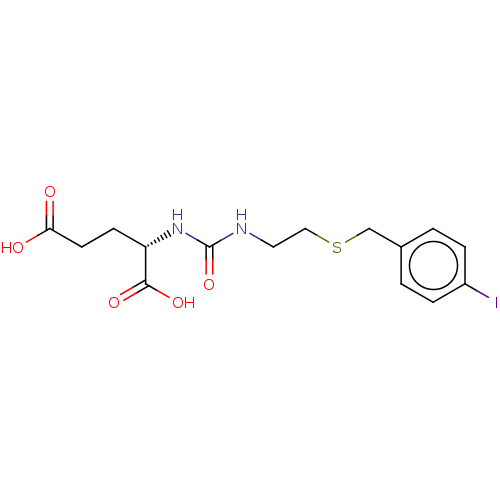
(US10736974, Compound DCIBC)Show SMILES OC(=O)CC[C@H](NC(=O)NCCSCc1ccc(I)cc1)C(O)=O |r| Show InChI InChI=1S/C15H19IN2O5S/c16-11-3-1-10(2-4-11)9-24-8-7-17-15(23)18-12(14(21)22)5-6-13(19)20/h1-4,12H,5-9H2,(H,19,20)(H,21,22)(H2,17,18,23)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM578399
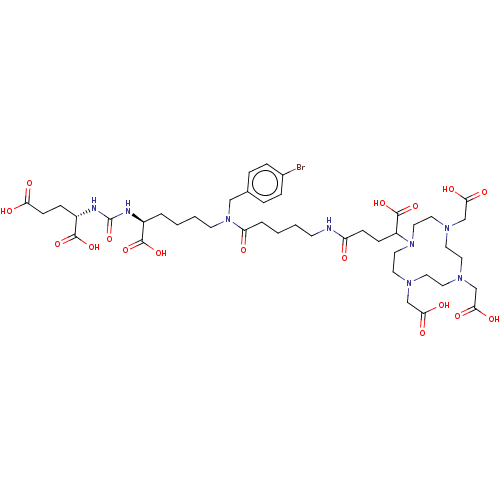
(US11478558, Compound L9)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCN(Cc1ccc(Br)cc1)C(=O)CCCCNC(=O)CCC(N1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(O)=O)C(O)=O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20P138Q |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456931

(US10736974, Compound XY-58)Show SMILES Cc1cc(Br)ccc1C(=O)NCCCC(=O)N[C@H](CNC(=O)N[C@@H](CCC(O)=O)C(O)=O)OC=O |r| Show InChI InChI=1S/C21H27BrN4O9/c1-12-9-13(22)4-5-14(12)19(31)23-8-2-3-16(28)26-17(35-11-27)10-24-21(34)25-15(20(32)33)6-7-18(29)30/h4-5,9,11,15,17H,2-3,6-8,10H2,1H3,(H,23,31)(H,26,28)(H,29,30)(H,32,33)(H2,24,25,34)/t15-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50102826
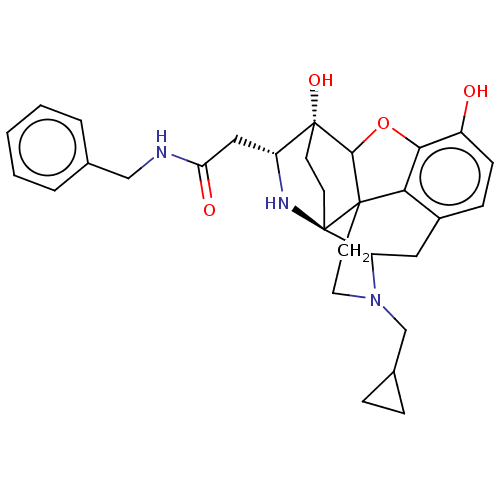
(CHEMBL3339374)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@@H]1CC(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,17:18:23:7.12.13,22:23:4.18.5:7.12.13,24:23:4.18.5:7.12.13,8:7:4.18.5:23| Show InChI InChI=1S/C30H35N3O4/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35)/t22-,23?,27?,28?,29-,30-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50491882
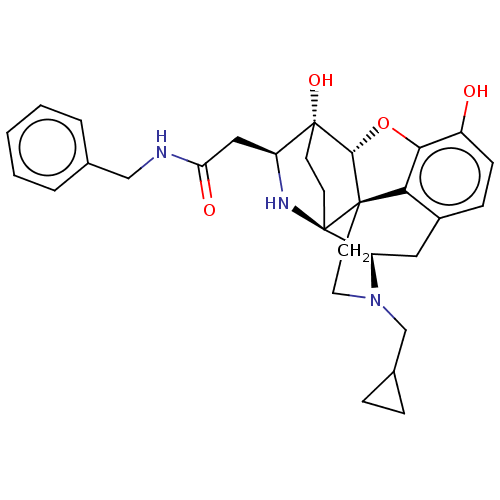
(CHEMBL3215908)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)NCc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C30H35N3O4.2ClH/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19;;/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35);2*1H/t22-,23+,27+,28+,29+,30+;;/m0../s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50102833
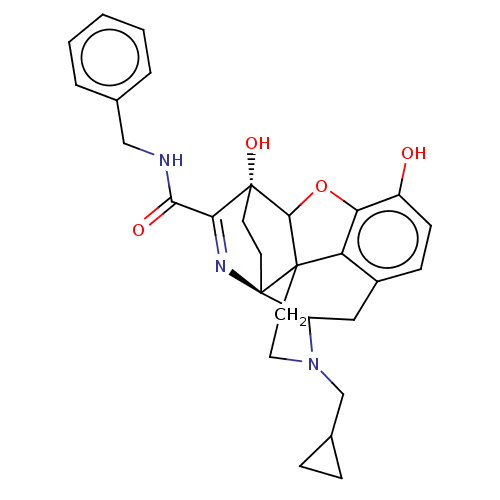
(CHEMBL3339378)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N=C1C(=O)NCc1ccccc1 |r,c:30,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,8:7:4.18.5:23,17:18:23:7.12.13,24:23:4.18.5:7.12.13,22:23:4.18.5:7.12.13| Show InChI InChI=1S/C29H31N3O4/c33-20-9-8-19-14-21-29-11-10-28(35,24(31-29)25(34)30-15-17-4-2-1-3-5-17)26-27(29,22(19)23(20)36-26)12-13-32(21)16-18-6-7-18/h1-5,8-9,18,21,26,33,35H,6-7,10-16H2,(H,30,34)/t21?,26?,27?,28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50380909
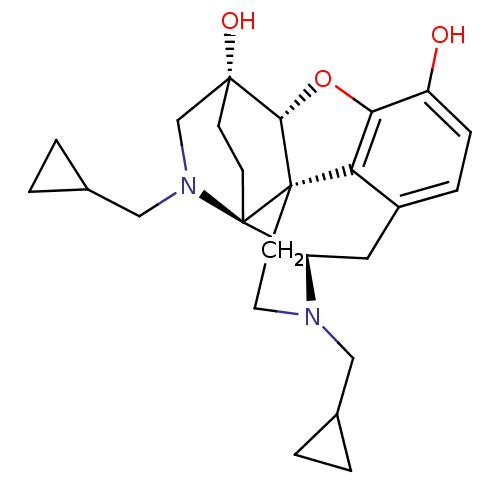
(CHEMBL2016678)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@]1(O)CC[C@@]35N(CC2CC2)C1 |r,THB:25:24:22.21:14.15| Show InChI InChI=1S/C25H32N2O3/c28-18-6-5-17-11-19-25-8-7-23(29,14-27(25)13-16-3-4-16)22-24(25,20(17)21(18)30-22)9-10-26(19)12-15-1-2-15/h5-6,15-16,19,22,28-29H,1-4,7-14H2/t19-,22+,23-,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mouse brain mu opioid receptor |
Bioorg Med Chem Lett 22: 2689-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.001
BindingDB Entry DOI: 10.7270/Q24X58T4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491882

(CHEMBL3215908)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)NCc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C30H35N3O4.2ClH/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19;;/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35);2*1H/t22-,23+,27+,28+,29+,30+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50102826

(CHEMBL3339374)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@@H]1CC(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,17:18:23:7.12.13,22:23:4.18.5:7.12.13,24:23:4.18.5:7.12.13,8:7:4.18.5:23| Show InChI InChI=1S/C30H35N3O4/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35)/t22-,23?,27?,28?,29-,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse brain membranes without cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM254931
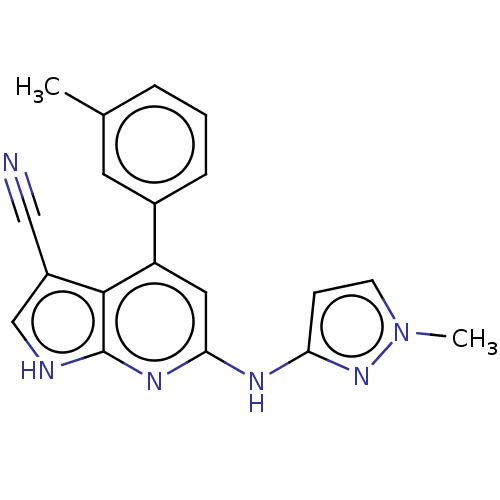
(US9499542, 14 | US9675594, 14)Show SMILES Cc1cccc(c1)-c1cc(Nc2ccn(C)n2)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C19H16N6/c1-12-4-3-5-13(8-12)15-9-17(22-16-6-7-25(2)24-16)23-19-18(15)14(10-20)11-21-19/h3-9,11H,1-2H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... |
J Med Chem 60: 8945-8962 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01186
BindingDB Entry DOI: 10.7270/Q2BR8VMR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491892

(CHEMBL3216338)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(O)[C@@H](N1)C(=O)Nc1ccccc1)ccc3O |r,THB:26:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C28H31N3O4.2ClH/c32-19-9-8-17-14-20-28-11-10-27(34,23(30-28)24(33)29-18-4-2-1-3-5-18)25-26(28,21(17)22(19)35-25)12-13-31(20)15-16-6-7-16;;/h1-5,8-9,16,20,23,25,30,32,34H,6-7,10-15H2,(H,29,33);2*1H/t20-,23+,25-,26+,27-,28-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491883

(CHEMBL3216579)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)Nc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C29H33N3O4.2ClH/c33-20-9-8-18-14-22-29-11-10-28(35,21(31-29)15-23(34)30-19-4-2-1-3-5-19)26-27(29,24(18)25(20)36-26)12-13-32(22)16-17-6-7-17;;/h1-5,8-9,17,21-22,26,31,33,35H,6-7,10-16H2,(H,30,34);2*1H/t21-,22+,26+,27+,28+,29+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391602

(CHEMBL2147915)Show SMILES CN1CC[C@]23Cc4nc5c(O)cccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H24N2O3/c1-26-8-7-23-13-19-16(9-15-3-2-4-20(28)22(15)25-19)12-24(23,29)21(26)10-14-5-6-17(27)11-18(14)23/h2-6,9,11,21,27-29H,7-8,10,12-13H2,1H3/t21-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50161762

(CHEMBL3785854)Show SMILES [H]C12Cc3ccc(O)cc3[C@@]3(CCN1CC1CC1)Cc1[nH]c4ccccc4c1C[C@@]23O |r,TLB:14:13:3.9.2:29| Show InChI InChI=1S/C26H28N2O2/c29-18-8-7-17-11-24-26(30)13-20-19-3-1-2-4-22(19)27-23(20)14-25(26,21(17)12-18)9-10-28(24)15-16-5-6-16/h1-4,7-8,12,16,24,27,29-30H,5-6,9-11,13-15H2/t24?,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0945 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes |
Bioorg Med Chem 24: 2199-205 (2016)
Article DOI: 10.1016/j.bmc.2016.03.040
BindingDB Entry DOI: 10.7270/Q2WW7KKZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50380906
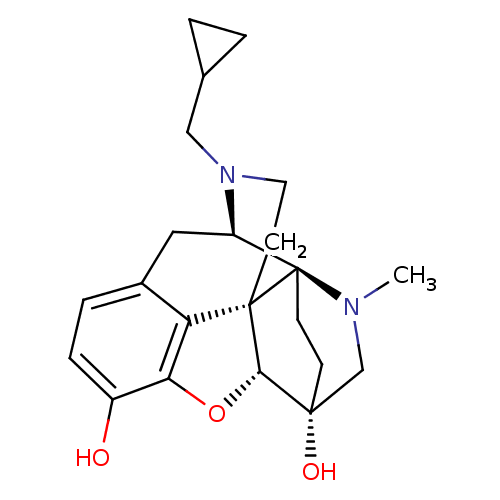
(CHEMBL2016675)Show SMILES CN1C[C@]2(O)CC[C@]11[C@H]3Cc4ccc(O)c5O[C@@H]2[C@]1(CCN3CC1CC1)c45 |r,TLB:0:1:6.5:18.17| Show InChI InChI=1S/C22H28N2O3/c1-23-12-20(26)6-7-22(23)16-10-14-4-5-15(25)18-17(14)21(22,19(20)27-18)8-9-24(16)11-13-2-3-13/h4-5,13,16,19,25-26H,2-3,6-12H2,1H3/t16-,19+,20-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mouse brain mu opioid receptor |
Bioorg Med Chem Lett 22: 2689-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.001
BindingDB Entry DOI: 10.7270/Q24X58T4 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM254931

(US9499542, 14 | US9675594, 14)Show SMILES Cc1cccc(c1)-c1cc(Nc2ccn(C)n2)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C19H16N6/c1-12-4-3-5-13(8-12)15-9-17(22-16-6-7-25(2)24-16)23-19-18(15)14(10-20)11-21-19/h3-9,11H,1-2H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... |
J Med Chem 60: 8945-8962 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01186
BindingDB Entry DOI: 10.7270/Q2BR8VMR |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM456929
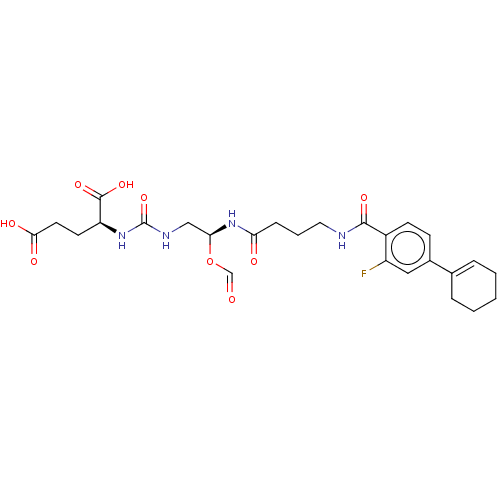
(US10736974, Compound XY-45)Show SMILES OC(=O)CC[C@H](NC(=O)NC[C@@H](NC(=O)CCCNC(=O)c1ccc(cc1F)C1=CCCCC1)OC=O)C(O)=O |r,t:29| Show InChI InChI=1S/C26H33FN4O9/c27-19-13-17(16-5-2-1-3-6-16)8-9-18(19)24(36)28-12-4-7-21(33)31-22(40-15-32)14-29-26(39)30-20(25(37)38)10-11-23(34)35/h5,8-9,13,15,20,22H,1-4,6-7,10-12,14H2,(H,28,36)(H,31,33)(H,34,35)(H,37,38)(H2,29,30,39)/t20-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY
US Patent
| Assay Description
The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... |
US Patent US10736974 (2020)
BindingDB Entry DOI: 10.7270/Q29C71GJ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582714

(CHEMBL5075978)Show SMILES C[C@@H]1CCCN1c1c(C#N)c2c(N)nc(Nc3cnn(C)c3)nc2n1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00720
BindingDB Entry DOI: 10.7270/Q2MP5754 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50253136
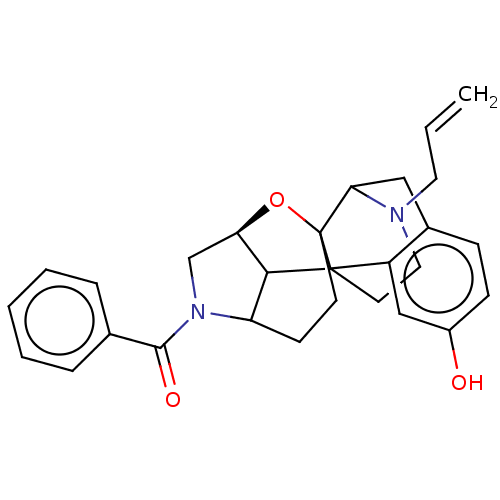
(CHEMBL4075409)Show SMILES [H][C@]12CN(C3CCC4(O1)C1Cc5ccc(O)cc5C4(CCN1CC=C)C23)C(=O)c1ccccc1 |r,TLB:3:4:18:8.1,2:1:18:5.6.4,6:7:21.20.19:10.17.11,17:18:8.1:5.6.4,16:17:21.20.19:7,THB:19:18:8.1:5.6.4| Show InChI InChI=1S/C28H30N2O3/c1-2-13-29-14-12-27-21-16-20(31)9-8-19(21)15-24(29)28(27)11-10-22-25(27)23(33-28)17-30(22)26(32)18-6-4-3-5-7-18/h2-9,16,22-25,31H,1,10-15,17H2/t22?,23-,24?,25?,27?,28?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1, Shirokane, Minato-ku, Tokyo 108-8641, Japan; Discovery Research Laboratories, Nippon Chemiphar Co., Ltd., 1-22, Hiko
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum membrane |
Bioorg Med Chem Lett 27: 2742-2745 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.059
BindingDB Entry DOI: 10.7270/Q2H134GP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50491883

(CHEMBL3216579)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)Nc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C29H33N3O4.2ClH/c33-20-9-8-18-14-22-29-11-10-28(35,21(31-29)15-23(34)30-19-4-2-1-3-5-19)26-27(29,24(18)25(20)36-26)12-13-32(22)16-17-6-7-17;;/h1-5,8-9,17,21-22,26,31,33,35H,6-7,10-16H2,(H,30,34);2*1H/t21-,22+,26+,27+,28+,29+;;/m0../s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50102828
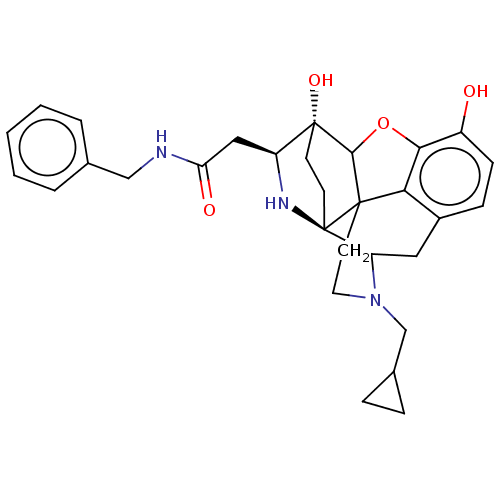
(CHEMBL3339372)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@H]1CC(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,17:18:23:7.12.13,22:23:4.18.5:7.12.13,24:23:4.18.5:7.12.13,8:7:4.18.5:23| Show InChI InChI=1S/C30H35N3O4/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35)/t22-,23?,27?,28?,29+,30+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50380911

(CHEMBL2016680)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@]1(O)CC[C@@]35O[C@@H]1C(=O)Nc1ccccc1 |r,THB:26:25:22.21:14.15| Show InChI InChI=1S/C28H30N2O5/c31-19-9-8-17-14-20-28-11-10-27(33,23(35-28)24(32)29-18-4-2-1-3-5-18)25-26(28,21(17)22(19)34-25)12-13-30(20)15-16-6-7-16/h1-5,8-9,16,20,23,25,31,33H,6-7,10-15H2,(H,29,32)/t20-,23-,25-,26+,27-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 22: 2689-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.001
BindingDB Entry DOI: 10.7270/Q24X58T4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491897

(CHEMBL2387739)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)Nc2ccccc2)N1C(C)=O)ccc3O |r,THB:34:18:5.7.6:16.10.15,35:34:2.17:19.20,4:5:18:16.10.15,24:23:2.17:19.20| Show InChI InChI=1S/C31H35N3O5.ClH/c1-18(35)34-23(16-25(37)32-21-5-3-2-4-6-21)30(38)11-12-31(34)24-15-20-9-10-22(36)27-26(20)29(31,28(30)39-27)13-14-33(24)17-19-7-8-19;/h2-6,9-10,19,23-24,28,36,38H,7-8,11-17H2,1H3,(H,32,37);1H/t23-,24+,28+,29+,30+,31+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50491884

(CHEMBL3216801)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@@H](CC(=O)NCc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C30H35N3O4.2ClH/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19;;/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35);2*1H/t22-,23-,27-,28-,29-,30-;;/m1../s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50491892

(CHEMBL3216338)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(O)[C@@H](N1)C(=O)Nc1ccccc1)ccc3O |r,THB:26:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C28H31N3O4.2ClH/c32-19-9-8-17-14-20-28-11-10-27(34,23(30-28)24(33)29-18-4-2-1-3-5-18)25-26(28,21(17)22(19)35-25)12-13-31(20)15-16-6-7-16;;/h1-5,8-9,16,20,23,25,30,32,34H,6-7,10-15H2,(H,29,33);2*1H/t20-,23+,25-,26+,27-,28-;;/m1../s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50118178
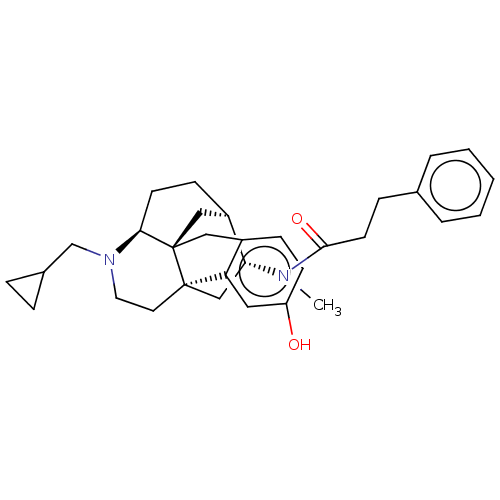
(CHEMBL3613172)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)CCc1ccccc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C32H40N2O2.ClH/c1-33(30(36)14-9-22-5-3-2-4-6-22)28-20-31-15-16-34(21-23-7-8-23)29-13-11-25(28)19-32(29,31)18-24-10-12-26(35)17-27(24)31;/h2-6,10,12,17,23,25,28-29,35H,7-9,11,13-16,18-21H2,1H3;1H/t25-,28-,29+,31-,32-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50102832
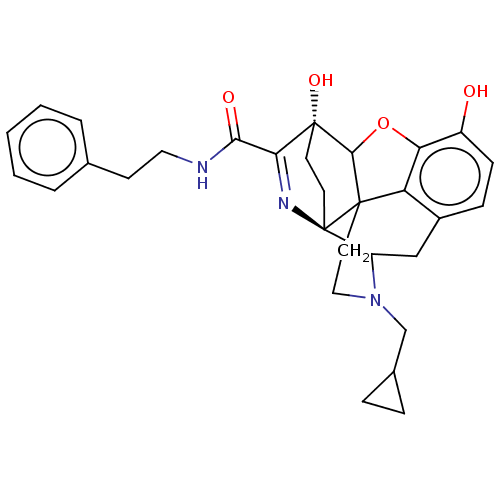
(CHEMBL3339379)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N=C1C(=O)NCCc1ccccc1 |r,c:30,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,8:7:4.18.5:23,17:18:23:7.12.13,24:23:4.18.5:7.12.13,22:23:4.18.5:7.12.13| Show InChI InChI=1S/C30H33N3O4/c34-21-9-8-20-16-22-30-12-11-29(36,25(32-30)26(35)31-14-10-18-4-2-1-3-5-18)27-28(30,23(20)24(21)37-27)13-15-33(22)17-19-6-7-19/h1-5,8-9,19,22,27,34,36H,6-7,10-17H2,(H,31,35)/t22?,27?,28?,29-,30-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50374600
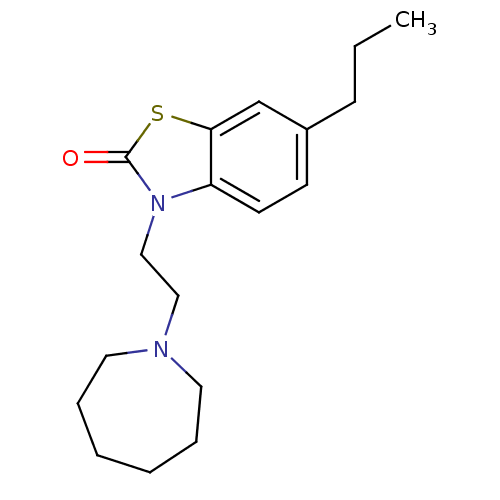
(CHEMBL272899)Show InChI InChI=1S/C18H26N2OS/c1-2-7-15-8-9-16-17(14-15)22-18(21)20(16)13-12-19-10-5-3-4-6-11-19/h8-9,14H,2-7,10-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by PDSP Ki Database
| Assay Description
Displacement of [3H](+)-pentazocine from opioid sigma1 receptor in rat brain homogenate |
J Med Chem 51: 1482-6 (2008)
Article DOI: 10.1021/jm701357m
BindingDB Entry DOI: 10.7270/Q2736RTZ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM578398
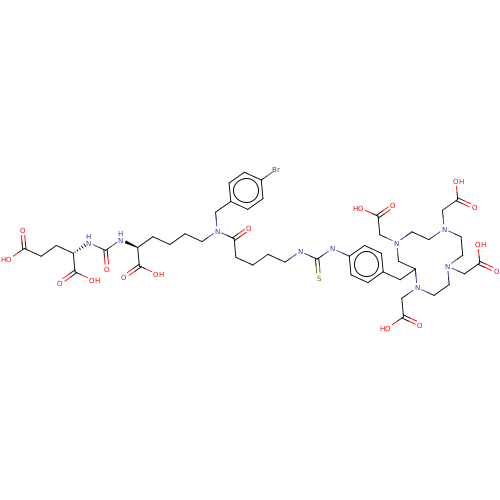
(US11478558, Compound L8)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCN(Cc1ccc(Br)cc1)C(=O)CCCCNC(=S)Nc1ccc(CC2CN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CCN2CC(O)=O)cc1)C(O)=O)C(O)=O |r,$;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;N;;;N;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;$| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20P138Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50380911

(CHEMBL2016680)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@]1(O)CC[C@@]35O[C@@H]1C(=O)Nc1ccccc1 |r,THB:26:25:22.21:14.15| Show InChI InChI=1S/C28H30N2O5/c31-19-9-8-17-14-20-28-11-10-27(33,23(35-28)24(32)29-18-4-2-1-3-5-18)25-26(28,21(17)22(19)34-25)12-13-30(20)15-16-6-7-16/h1-5,8-9,16,20,23,25,31,33H,6-7,10-15H2,(H,29,32)/t20-,23-,25-,26+,27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50360146
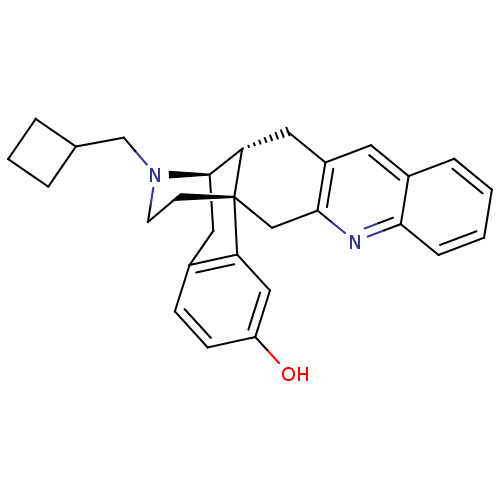
(CHEMBL1929368)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4Cc5cc6ccccc6nc5C[C@]4(CCN3CC3CCC3)c2c1 |r,THB:24:23:7:29.4.5| Show InChI InChI=1S/C28H30N2O/c31-22-9-8-19-14-27-24-13-21-12-20-6-1-2-7-25(20)29-26(21)16-28(24,23(19)15-22)10-11-30(27)17-18-4-3-5-18/h1-2,6-9,12,15,18,24,27,31H,3-5,10-11,13-14,16-17H2/t24-,27+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 20: 949-61 (2012)
Article DOI: 10.1016/j.bmc.2011.11.047
BindingDB Entry DOI: 10.7270/Q2H995MX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50599576

(CHEMBL5200218)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)CCc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491884

(CHEMBL3216801)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@@H](CC(=O)NCc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C30H35N3O4.2ClH/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19;;/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35);2*1H/t22-,23-,27-,28-,29-,30-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50274482
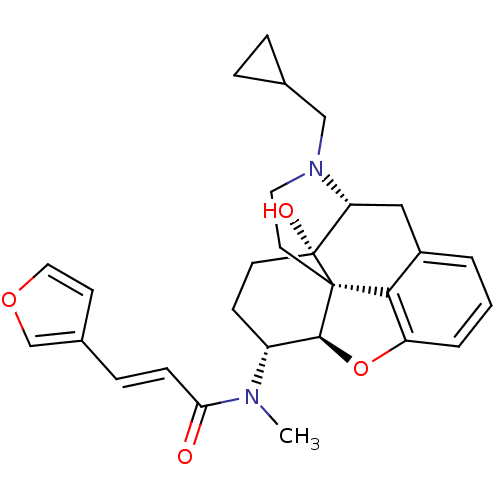
((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-1...)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4cccc5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 |r,TLB:4:5:9.24.8:17.19.18,THB:6:5:9.24.8:17.19.18| Show InChI InChI=1S/C28H32N2O4/c1-29(24(31)8-7-19-10-14-33-17-19)21-9-11-28(32)23-15-20-3-2-4-22-25(20)27(28,26(21)34-22)12-13-30(23)16-18-5-6-18/h2-4,7-8,10,14,17-18,21,23,26,32H,5-6,9,11-13,15-16H2,1H3/b8-7+/t21-,23-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50102828

(CHEMBL3339372)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@H]1CC(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,17:18:23:7.12.13,22:23:4.18.5:7.12.13,24:23:4.18.5:7.12.13,8:7:4.18.5:23| Show InChI InChI=1S/C30H35N3O4/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35)/t22-,23?,27?,28?,29+,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse brain membranes without cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491885
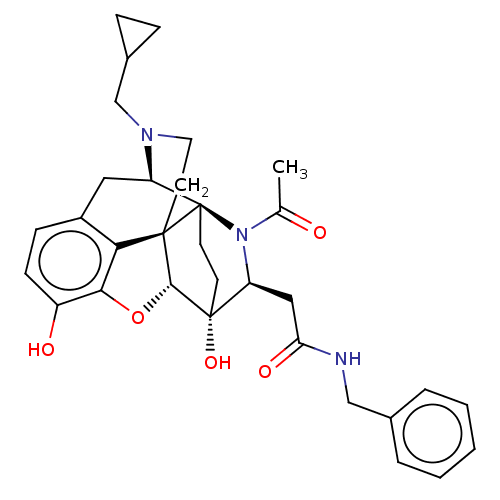
(CHEMBL2387740)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)NCc2ccccc2)N1C(C)=O)ccc3O |r,THB:35:18:5.7.6:16.10.15,4:5:18:16.10.15,24:23:2.17:19.20,36:35:2.17:19.20| Show InChI InChI=1S/C32H37N3O5.ClH/c1-19(36)35-24(16-26(38)33-17-20-5-3-2-4-6-20)31(39)11-12-32(35)25-15-22-9-10-23(37)28-27(22)30(32,29(31)40-28)13-14-34(25)18-21-7-8-21;/h2-6,9-10,21,24-25,29,37,39H,7-8,11-18H2,1H3,(H,33,38);1H/t24-,25+,29+,30+,31+,32+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642632

(US20230416245, Compound 70)Show SMILES CCNc1cc(nc2n(cnc12)[C@@H]1[C@H]2C[C@@]2([C@@H](O)[C@H]1O)C(=O)NC)C#N |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50118191

(CHEMBL3613173)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)Cc1ccccc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C31H38N2O2.ClH/c1-32(29(35)15-21-5-3-2-4-6-21)27-19-30-13-14-33(20-22-7-8-22)28-12-10-24(27)18-31(28,30)17-23-9-11-25(34)16-26(23)30;/h2-6,9,11,16,22,24,27-28,34H,7-8,10,12-15,17-20H2,1H3;1H/t24-,27-,28+,30-,31-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50294428
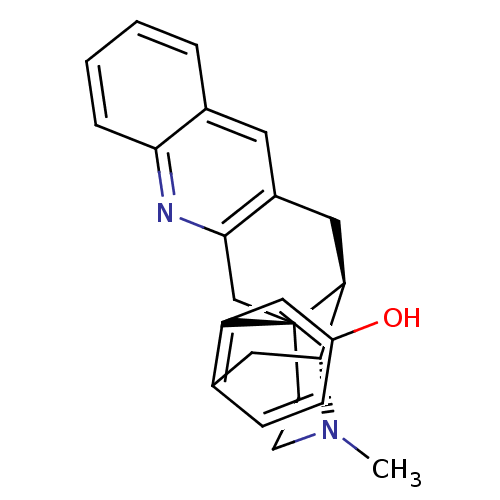
(CHEMBL552308 | SN-28)Show SMILES CN1CC[C@]23Cc4nc5ccccc5cc4C[C@H]2[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:17:26.20.19| Show InChI InChI=1S/C24H24N2O/c1-26-9-8-24-14-22-17(10-16-4-2-3-5-21(16)25-22)11-20(24)23(26)12-15-6-7-18(27)13-19(15)24/h2-7,10,13,20,23,27H,8-9,11-12,14H2,1H3/t20-,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain membranes |
Bioorg Med Chem Lett 20: 6302-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.083
BindingDB Entry DOI: 10.7270/Q2G73DZ1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50102833

(CHEMBL3339378)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N=C1C(=O)NCc1ccccc1 |r,c:30,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,8:7:4.18.5:23,17:18:23:7.12.13,24:23:4.18.5:7.12.13,22:23:4.18.5:7.12.13| Show InChI InChI=1S/C29H31N3O4/c33-20-9-8-19-14-21-29-11-10-28(35,24(31-29)25(34)30-15-17-4-2-1-3-5-17)26-27(29,22(19)23(20)36-26)12-13-32(21)16-18-6-7-18/h1-5,8-9,18,21,26,33,35H,6-7,10-16H2,(H,30,34)/t21?,26?,27?,28-,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse brain membranes without cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50329100
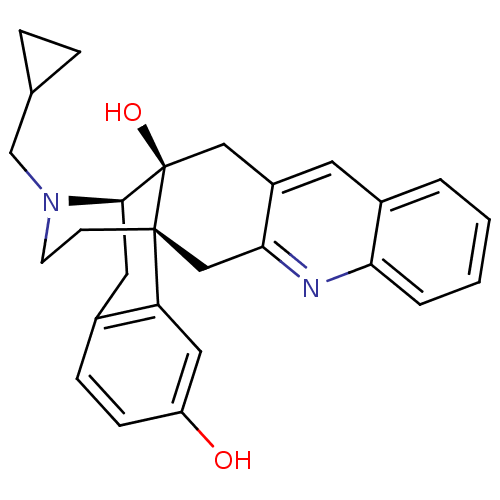
(CHEMBL1270475)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]4(Cc5nc6ccccc6cc5C[C@@]34O)c2c1 |r,THB:8:7:27:4.29.5| Show InChI InChI=1S/C27H28N2O2/c30-21-8-7-18-12-25-27(31)14-20-11-19-3-1-2-4-23(19)28-24(20)15-26(27,22(18)13-21)9-10-29(25)16-17-5-6-17/h1-4,7-8,11,13,17,25,30-31H,5-6,9-10,12,14-16H2/t25-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain membranes |
Bioorg Med Chem Lett 20: 6302-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.083
BindingDB Entry DOI: 10.7270/Q2G73DZ1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50294428

(CHEMBL552308 | SN-28)Show SMILES CN1CC[C@]23Cc4nc5ccccc5cc4C[C@H]2[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:17:26.20.19| Show InChI InChI=1S/C24H24N2O/c1-26-9-8-24-14-22-17(10-16-4-2-3-5-21(16)25-22)11-20(24)23(26)12-15-6-7-18(27)13-19(15)24/h2-7,10,13,20,23,27H,8-9,11-12,14H2,1H3/t20-,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from delta opioid receptor in rat cerebrum membranes |
Bioorg Med Chem 20: 949-61 (2012)
Article DOI: 10.1016/j.bmc.2011.11.047
BindingDB Entry DOI: 10.7270/Q2H995MX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599574
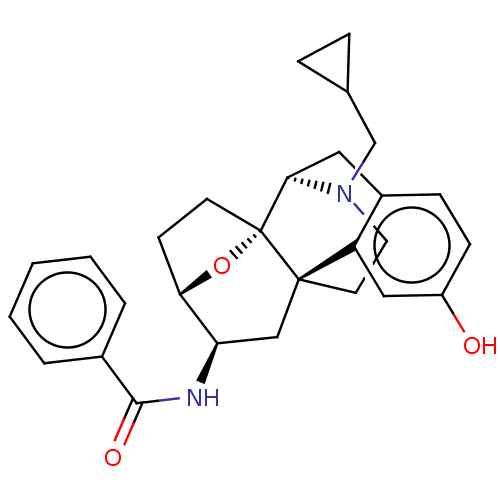
(CHEMBL5186730)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)c1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50494888

(CHEMBL4296733)Show SMILES Cl.Cl.[H][C@]12Cc3ccc(O)cc3[C@@]3(CCN1CC)Cc1nc4ccccc4cc1C[C@@]23O |r,TLB:6:5:30:15.14.13,THB:10:11:30:15.14.13| Show InChI InChI=1S/C25H26N2O2.2ClH/c1-2-27-10-9-24-15-22-18(11-17-5-3-4-6-21(17)26-22)14-25(24,29)23(27)12-16-7-8-19(28)13-20(16)24;;/h3-8,11,13,23,28-29H,2,9-10,12,14-15H2,1H3;2*1H/t23-,24-,25-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPDPE from delta opioid receptor in mouse whole brain membranes without cerebellum after 1 hr by liquid scintillation counting a... |
Bioorg Med Chem 21: 7628-47 (2013)
Article DOI: 10.1016/j.bmc.2013.10.032
BindingDB Entry DOI: 10.7270/Q2VM4G71 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391591
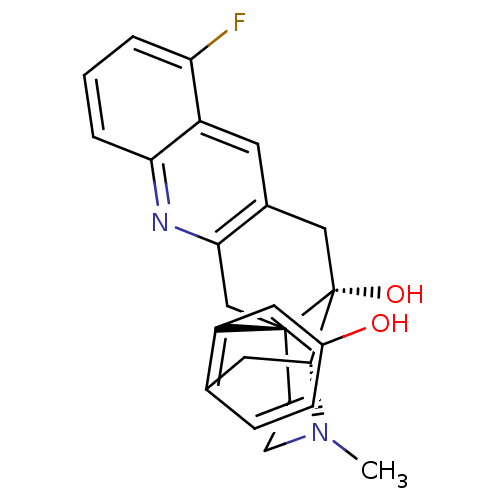
(CHEMBL2147904)Show SMILES CN1CC[C@]23Cc4nc5cccc(F)c5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H23FN2O2/c1-27-8-7-23-13-21-15(9-17-19(25)3-2-4-20(17)26-21)12-24(23,29)22(27)10-14-5-6-16(28)11-18(14)23/h2-6,9,11,22,28-29H,7-8,10,12-13H2,1H3/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50329100

(CHEMBL1270475)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]4(Cc5nc6ccccc6cc5C[C@@]34O)c2c1 |r,THB:8:7:27:4.29.5| Show InChI InChI=1S/C27H28N2O2/c30-21-8-7-18-12-25-27(31)14-20-11-19-3-1-2-4-23(19)28-24(20)15-26(27,22(18)13-21)9-10-29(25)16-17-5-6-17/h1-4,7-8,11,13,17,25,30-31H,5-6,9-10,12,14-16H2/t25-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DPDPE from delta opioid receptor in mouse whole brain membranes without cerebellum after 1 hr by liquid scintillation counting a... |
Bioorg Med Chem 21: 7628-47 (2013)
Article DOI: 10.1016/j.bmc.2013.10.032
BindingDB Entry DOI: 10.7270/Q2VM4G71 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50391594
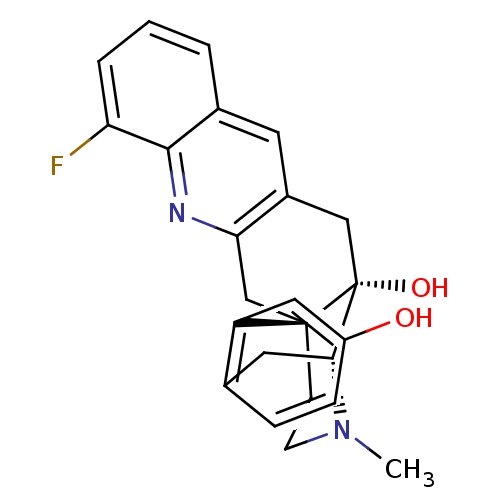
(CHEMBL2147907)Show SMILES CN1CC[C@]23Cc4nc5c(F)cccc5cc4C[C@@]2(O)[C@H]1Cc1ccc(O)cc31 |r,THB:0:1:18:28.22.21| Show InChI InChI=1S/C24H23FN2O2/c1-27-8-7-23-13-20-16(9-15-3-2-4-19(25)22(15)26-20)12-24(23,29)21(27)10-14-5-6-17(28)11-18(14)23/h2-6,9,11,21,28-29H,7-8,10,12-13H2,1H3/t21-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from delta opioid receptor in mouse whole brain without cerebellum |
Bioorg Med Chem 20: 5810-31 (2012)
Article DOI: 10.1016/j.bmc.2012.08.004
BindingDB Entry DOI: 10.7270/Q2F190T5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data