

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50220136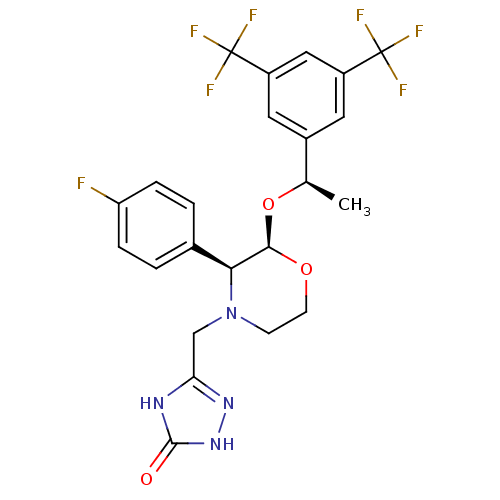 (3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description compounds were evaluated for inhibitory activity against human Tachykinin receptor 1 | J Med Chem 43: 1234-41 (2000) BindingDB Entry DOI: 10.7270/Q2G73CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM22857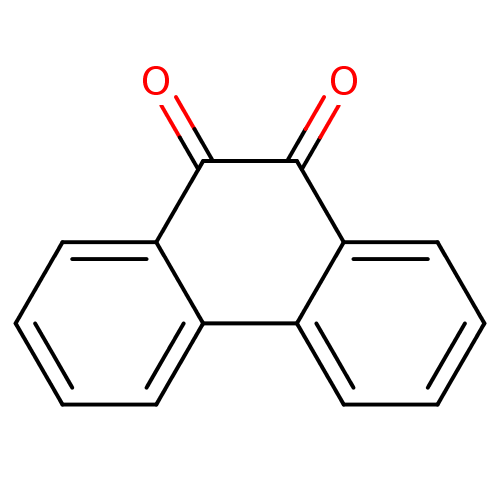 (1,2-Dione-Based Compound, 14 | 9,10-dihydrophenant...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107001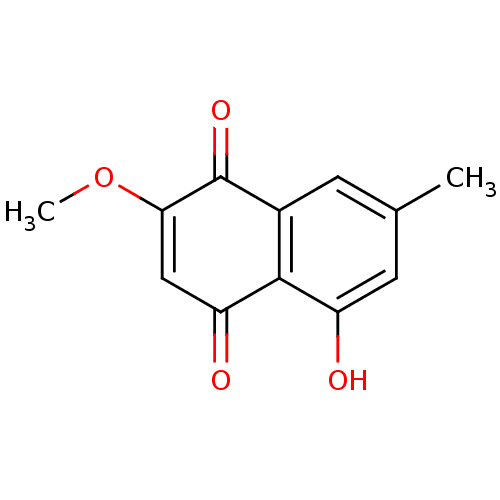 (2-methoxy-7-methyljuglone | 5-Hydroxy-2-methoxy-7-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107000 (6-Acetyl-5-hydroxy-2-methoxy-7-methyl-[1,4]naphtho...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107006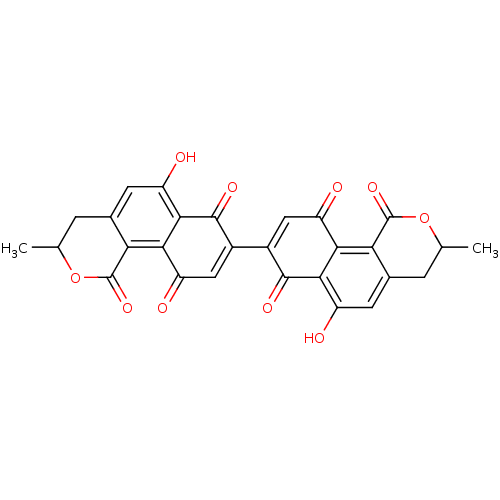 (9,9'-Dihydroxy-2,2'-dimethyl-1,2,1',2'-tetrahydro-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107009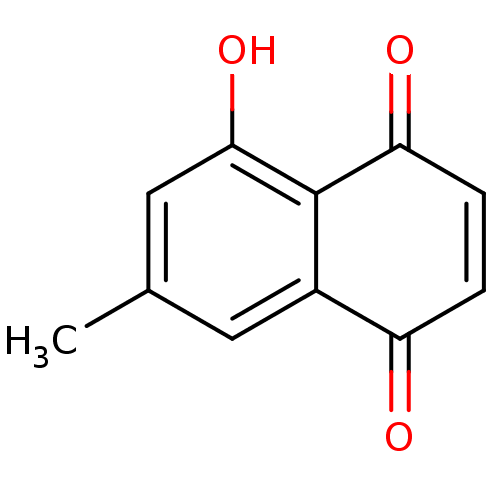 (5-Hydroxy-7-methyl-[1,4]naphthoquinone | 5-hydroxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107007 (Acetic acid 2-acetyl-6-methoxy-3-methyl-5,8-dioxo-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM22851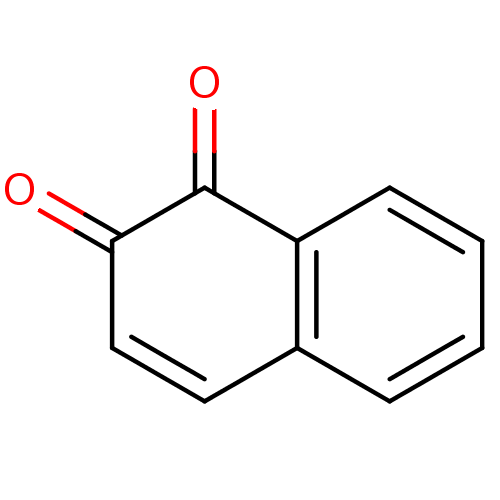 (1,2-Dione-Based Compound, 8 | 1,2-dihydronaphthale...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107005 (2-Methoxy-7-methyl-[1,4]naphthoquinone | CHEMBL106...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50107004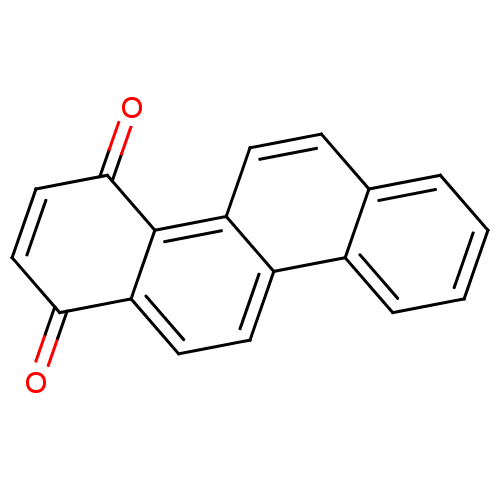 (CHEMBL421215 | Chrysene-1,4-dione) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against papain using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM24776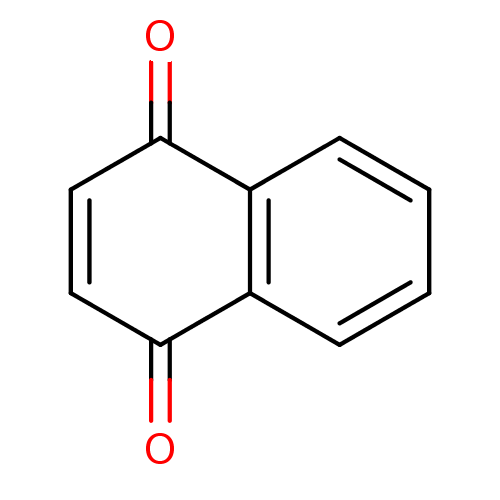 (1,4-Naphthoquinone | 1,4-Naphthoquinone (5a) | 1,4...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50107000 (6-Acetyl-5-hydroxy-2-methoxy-7-methyl-[1,4]naphtho...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against papain using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM24775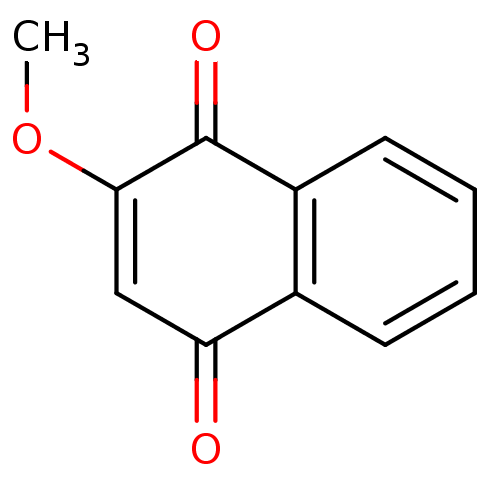 (2-methoxy-1,4-dihydronaphthalene-1,4-dione | 2-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM24776 (1,4-Naphthoquinone | 1,4-Naphthoquinone (5a) | 1,4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against papain using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50107007 (Acetic acid 2-acetyl-6-methoxy-3-methyl-5,8-dioxo-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against papain using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107003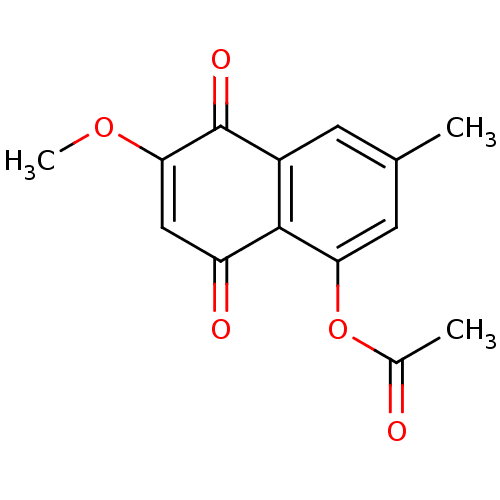 (Acetic acid 6-methoxy-3-methyl-5,8-dioxo-5,8-dihyd...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107004 (CHEMBL421215 | Chrysene-1,4-dione) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107010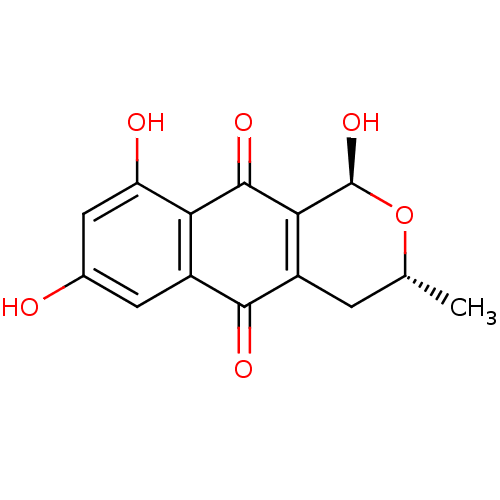 ((1S,3R)-1,7,9-Trihydroxy-3-methyl-3,4-dihydro-1H-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM22857 (1,2-Dione-Based Compound, 14 | 9,10-dihydrophenant...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against papain using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM22851 (1,2-Dione-Based Compound, 8 | 1,2-dihydronaphthale...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against papain using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107008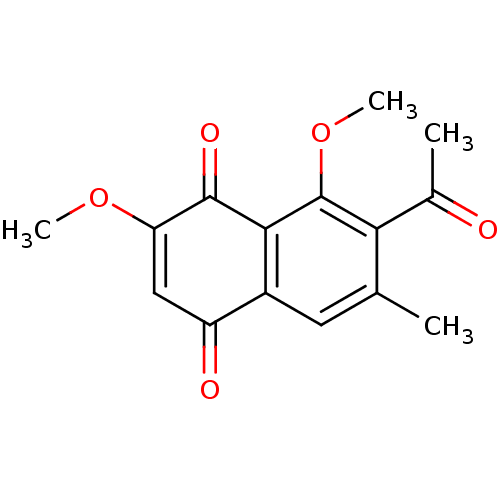 (7-Acetyl-2,8-dimethoxy-6-methyl-[1,4]naphthoquinon...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM11318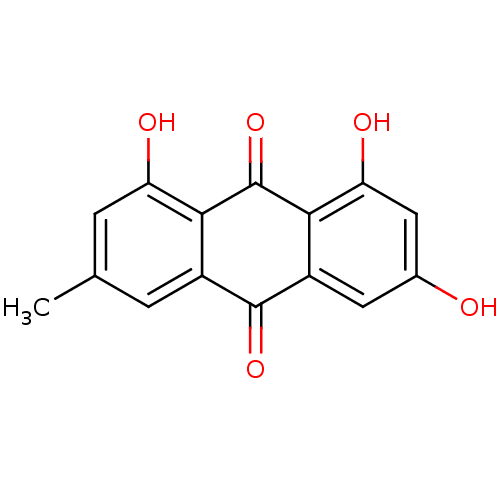 (1,3,8-trihydroxy-6-methyl-9,10-dihydroanthracene-9...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50005886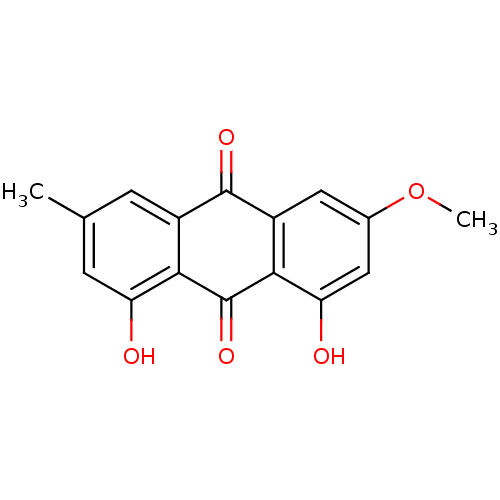 (1,8-Dihydroxy-3-methoxy-6-methylanthraquinone | 1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50107002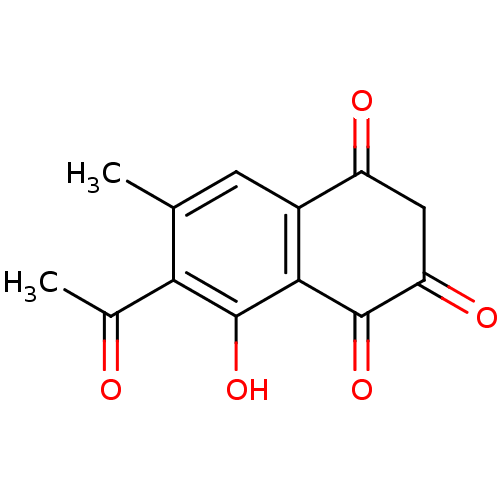 (7-Acetyl-2,8-dihydroxy-6-methyl-[1,4]naphthoquinon...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM24779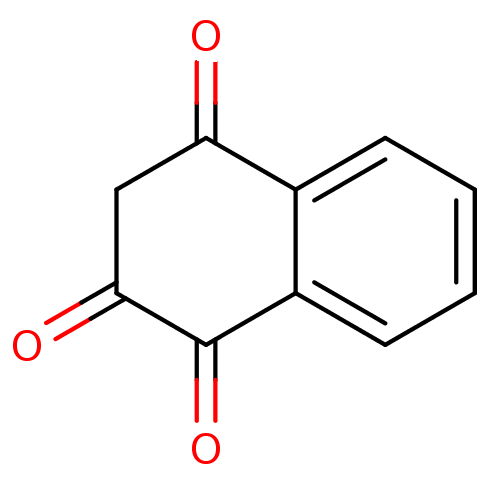 (2-Hydroxy-[1,4]naphthoquinone | 2-hydroxy-1,4-dihy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HRV 3Cpro using HPLC assay | Bioorg Med Chem Lett 11: 3143-6 (2001) BindingDB Entry DOI: 10.7270/Q2T43SDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||