 Found 86 hits with Last Name = 'reid' and Initial = 'g'
Found 86 hits with Last Name = 'reid' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13465

((2S)-N-[(1S)-1-carbamoyl-2-{4-[(5S)-1,1,3-trioxo-1...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)[C@@H]1CC(=O)NS1(=O)=O)C(N)=O |r| Show InChI InChI=1S/C23H26N4O6S/c1-14(28)25-19(12-15-5-3-2-4-6-15)23(31)26-18(22(24)30)11-16-7-9-17(10-8-16)20-13-21(29)27-34(20,32)33/h2-10,18-20H,11-13H2,1H3,(H2,24,30)(H,25,28)(H,26,31)(H,27,29)/t18-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant against protein-tyrosine phosphatase 1B by PNPP enzyme assay |
J Med Chem 48: 6544-8 (2005)
Article DOI: 10.1021/jm0504555
BindingDB Entry DOI: 10.7270/Q2805252 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185362

(US9156831, 3)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CCO Show InChI InChI=1S/C22H31N9O3/c1-5-31-19(26-18(29-31)13-6-9-30(10-7-13)15(33)8-11-32)14-12-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h12-13,32H,5-11H2,1-4H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185358

(US9156831, 9)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CC(O)=O Show InChI InChI=1S/C22H29N9O4/c1-5-31-19(26-18(29-31)12-6-8-30(9-7-12)14(32)10-15(33)34)13-11-24-17(23)16(25-13)20-27-28-21(35-20)22(2,3)4/h11-12H,5-10H2,1-4H3,(H2,23,24)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185362

(US9156831, 3)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CCO Show InChI InChI=1S/C22H31N9O3/c1-5-31-19(26-18(29-31)13-6-9-30(10-7-13)15(33)8-11-32)14-12-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h12-13,32H,5-11H2,1-4H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185358

(US9156831, 9)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CC(O)=O Show InChI InChI=1S/C22H29N9O4/c1-5-31-19(26-18(29-31)12-6-8-30(9-7-12)14(32)10-15(33)34)13-11-24-17(23)16(25-13)20-27-28-21(35-20)22(2,3)4/h11-12H,5-10H2,1-4H3,(H2,23,24)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185357

(US9156831, 8)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CC(O)=O Show InChI InChI=1S/C21H27N9O4/c1-21(2,3)20-27-26-19(34-20)15-16(22)23-10-12(24-15)18-25-17(28-29(18)4)11-5-7-30(8-6-11)13(31)9-14(32)33/h10-11H,5-9H2,1-4H3,(H2,22,23)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185354

(US9156831, 5)Show SMILES C[C@H](O)CC(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(32)10-15(33)31-8-6-13(7-9-31)18-26-19(30(5)29-18)14-11-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h11-13,32H,6-10H2,1-5H3,(H2,23,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185355
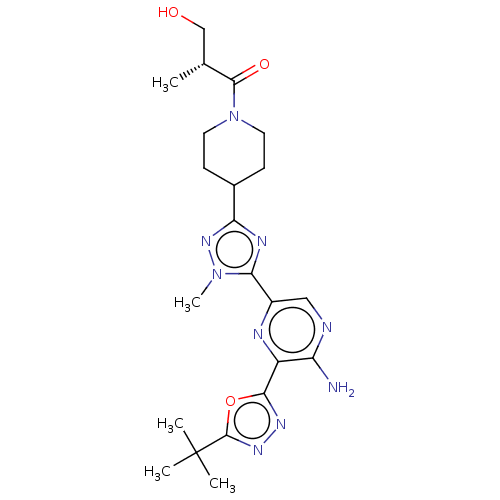
(US9156831, 6)Show SMILES C[C@H](CO)C(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(11-32)20(33)31-8-6-13(7-9-31)17-26-18(30(5)29-17)14-10-24-16(23)15(25-14)19-27-28-21(34-19)22(2,3)4/h10,12-13,32H,6-9,11H2,1-5H3,(H2,23,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <12 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185353

(US9156831, 4)Show SMILES C[C@@H](O)CC(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(32)10-15(33)31-8-6-13(7-9-31)18-26-19(30(5)29-18)14-11-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h11-13,32H,6-10H2,1-5H3,(H2,23,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <12 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185361

(US9156831, 1)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CCO Show InChI InChI=1S/C21H29N9O3/c1-21(2,3)20-27-26-19(33-20)15-16(22)23-11-13(24-15)18-25-17(28-29(18)4)12-5-8-30(9-6-12)14(32)7-10-31/h11-12,31H,5-10H2,1-4H3,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <14 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185356
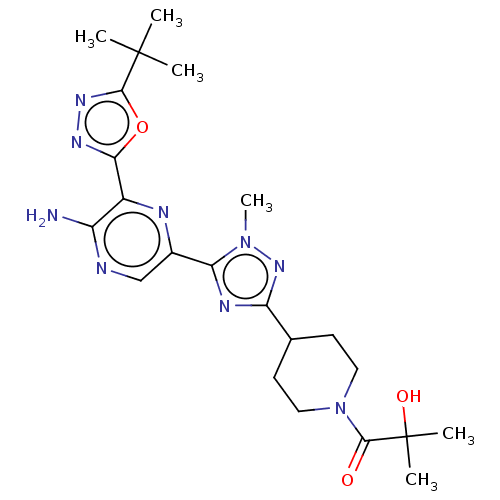
(US9156831, 7)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)C(C)(C)O Show InChI InChI=1S/C22H31N9O3/c1-21(2,3)19-28-27-18(34-19)14-15(23)24-11-13(25-14)17-26-16(29-30(17)6)12-7-9-31(10-8-12)20(32)22(4,5)33/h11-12,33H,7-10H2,1-6H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <14 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185361

(US9156831, 1)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CCO Show InChI InChI=1S/C21H29N9O3/c1-21(2,3)20-27-26-19(33-20)15-16(22)23-11-13(24-15)18-25-17(28-29(18)4)12-5-8-30(9-6-12)14(32)7-10-31/h11-12,31H,5-10H2,1-4H3,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185357

(US9156831, 8)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CC(O)=O Show InChI InChI=1S/C21H27N9O4/c1-21(2,3)20-27-26-19(34-20)15-16(22)23-10-12(24-15)18-25-17(28-29(18)4)11-5-7-30(8-6-11)13(31)9-14(32)33/h10-11H,5-9H2,1-4H3,(H2,22,23)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185353

(US9156831, 4)Show SMILES C[C@@H](O)CC(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(32)10-15(33)31-8-6-13(7-9-31)18-26-19(30(5)29-18)14-11-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h11-13,32H,6-10H2,1-5H3,(H2,23,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185354

(US9156831, 5)Show SMILES C[C@H](O)CC(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(32)10-15(33)31-8-6-13(7-9-31)18-26-19(30(5)29-18)14-11-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h11-13,32H,6-10H2,1-5H3,(H2,23,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185355

(US9156831, 6)Show SMILES C[C@H](CO)C(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(11-32)20(33)31-8-6-13(7-9-31)17-26-18(30(5)29-17)14-10-24-16(23)15(25-14)19-27-28-21(34-19)22(2,3)4/h10,12-13,32H,6-9,11H2,1-5H3,(H2,23,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185356

(US9156831, 7)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)C(C)(C)O Show InChI InChI=1S/C22H31N9O3/c1-21(2,3)19-28-27-18(34-19)14-15(23)24-11-13(25-14)17-26-16(29-30(17)6)12-7-9-31(10-8-12)20(32)22(4,5)33/h11-12,33H,7-10H2,1-6H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13809
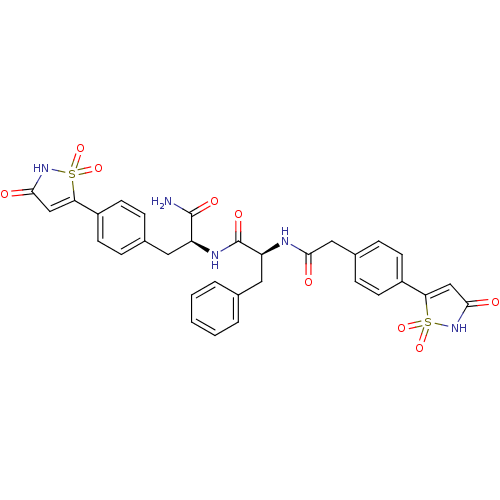
((2S)-N-[(1S)-1-carbamoyl-2-[4-(1,1,3-trioxo-2,3-di...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C1=CC(=O)NS1(=O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(cc1)C1=CC(=O)NS1(=O)=O |r,t:12,44| Show InChI InChI=1S/C32H29N5O9S2/c33-31(41)24(14-20-6-10-22(11-7-20)26-17-29(39)36-47(26,43)44)35-32(42)25(15-19-4-2-1-3-5-19)34-28(38)16-21-8-12-23(13-9-21)27-18-30(40)37-48(27,45)46/h1-13,17-18,24-25H,14-16H2,(H2,33,41)(H,34,38)(H,35,42)(H,36,39)(H,37,40)/t24-,25-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation
| Assay Description
The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... |
J Biol Chem 281: 32784-95 (2006)
Article DOI: 10.1074/jbc.M606873200
BindingDB Entry DOI: 10.7270/Q2GX48SD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM185363

(US9156831, 10)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)[C@@H](O)CO Show InChI InChI=1S/C22H31N9O4/c1-5-31-18(26-17(29-31)12-6-8-30(9-7-12)20(34)14(33)11-32)13-10-24-16(23)15(25-13)19-27-28-21(35-19)22(2,3)4/h10,12,14,32-33H,5-9,11H2,1-4H3,(H2,23,24)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | 37 |
AstraZeneca AB
US Patent
| Assay Description
This assay was used to measure PI3K-alpha inhibition in cells. BT474 cells (human breast ductal carcinoma, ATCC HTB-20) were seeded into black 384 we... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM185362

(US9156831, 3)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CCO Show InChI InChI=1S/C22H31N9O3/c1-5-31-19(26-18(29-31)13-6-9-30(10-7-13)15(33)8-11-32)14-12-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h12-13,32H,5-11H2,1-4H3,(H2,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | 37 |
AstraZeneca AB
US Patent
| Assay Description
This assay was used to measure PI3K-alpha inhibition in cells. BT474 cells (human breast ductal carcinoma, ATCC HTB-20) were seeded into black 384 we... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM185364
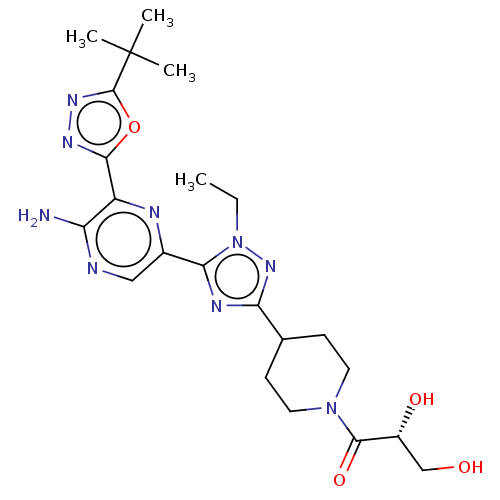
(US9156831, 11)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)[C@H](O)CO Show InChI InChI=1S/C22H31N9O4/c1-5-31-18(26-17(29-31)12-6-8-30(9-7-12)20(34)14(33)11-32)13-10-24-16(23)15(25-13)19-27-28-21(35-19)22(2,3)4/h10,12,14,32-33H,5-9,11H2,1-4H3,(H2,23,24)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | 37 |
AstraZeneca AB
US Patent
| Assay Description
This assay was used to measure PI3K-alpha inhibition in cells. BT474 cells (human breast ductal carcinoma, ATCC HTB-20) were seeded into black 384 we... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13465

((2S)-N-[(1S)-1-carbamoyl-2-{4-[(5S)-1,1,3-trioxo-1...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)[C@@H]1CC(=O)NS1(=O)=O)C(N)=O |r| Show InChI InChI=1S/C23H26N4O6S/c1-14(28)25-19(12-15-5-3-2-4-6-15)23(31)26-18(22(24)30)11-16-7-9-17(10-8-16)20-13-21(29)27-34(20,32)33/h2-10,18-20H,11-13H2,1H3,(H2,24,30)(H,25,28)(H,26,31)(H,27,29)/t18-,19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibitory concentration against T cell protein tyrosine phosphatase |
J Med Chem 48: 6544-8 (2005)
Article DOI: 10.1021/jm0504555
BindingDB Entry DOI: 10.7270/Q2805252 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13808
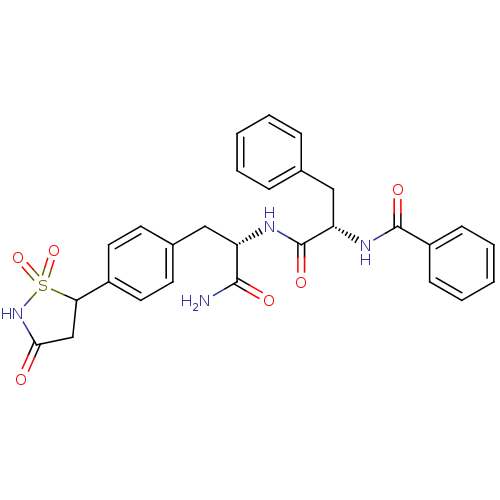
((2S)-N-[(1S)-1-carbamoyl-2-[4-(1,1,3-trioxo-1,2-th...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 |r| Show InChI InChI=1S/C28H28N4O6S/c29-26(34)22(15-19-11-13-20(14-12-19)24-17-25(33)32-39(24,37)38)30-28(36)23(16-18-7-3-1-4-8-18)31-27(35)21-9-5-2-6-10-21/h1-14,22-24H,15-17H2,(H2,29,34)(H,30,36)(H,31,35)(H,32,33)/t22-,23-,24?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation
| Assay Description
The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... |
J Biol Chem 281: 32784-95 (2006)
Article DOI: 10.1074/jbc.M606873200
BindingDB Entry DOI: 10.7270/Q2GX48SD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13465

((2S)-N-[(1S)-1-carbamoyl-2-{4-[(5S)-1,1,3-trioxo-1...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)[C@@H]1CC(=O)NS1(=O)=O)C(N)=O |r| Show InChI InChI=1S/C23H26N4O6S/c1-14(28)25-19(12-15-5-3-2-4-6-15)23(31)26-18(22(24)30)11-16-7-9-17(10-8-16)20-13-21(29)27-34(20,32)33/h2-10,18-20H,11-13H2,1H3,(H2,24,30)(H,25,28)(H,26,31)(H,27,29)/t18-,19-,20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation
| Assay Description
The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... |
J Biol Chem 281: 32784-95 (2006)
Article DOI: 10.1074/jbc.M606873200
BindingDB Entry DOI: 10.7270/Q2GX48SD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13465

((2S)-N-[(1S)-1-carbamoyl-2-{4-[(5S)-1,1,3-trioxo-1...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)[C@@H]1CC(=O)NS1(=O)=O)C(N)=O |r| Show InChI InChI=1S/C23H26N4O6S/c1-14(28)25-19(12-15-5-3-2-4-6-15)23(31)26-18(22(24)30)11-16-7-9-17(10-8-16)20-13-21(29)27-34(20,32)33/h2-10,18-20H,11-13H2,1H3,(H2,24,30)(H,25,28)(H,26,31)(H,27,29)/t18-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein-tyrosine phosphatase 1B by PNPP enzyme assay |
J Med Chem 48: 6544-8 (2005)
Article DOI: 10.1021/jm0504555
BindingDB Entry DOI: 10.7270/Q2805252 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ATP-binding cassette sub-family C member 4
(Homo sapiens (Human)) | BDBM50270011
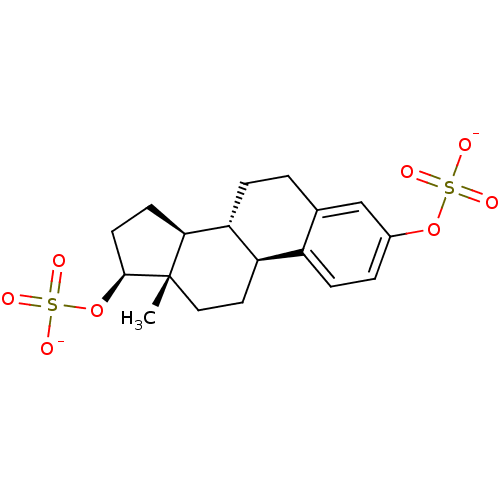
(estradiol disulfate)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS([O-])(=O)=O)ccc34)[C@@H]1CC[C@@H]2OS([O-])(=O)=O |r| Show InChI InChI=1S/C18H24O8S2/c1-18-9-8-14-13-5-3-12(25-27(19,20)21)10-11(13)2-4-15(14)16(18)6-7-17(18)26-28(22,23)24/h3,5,10,14-17H,2,4,6-9H2,1H3,(H,19,20,21)(H,22,23,24)/p-2/t14-,15-,16+,17+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Cancer Institute
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of DHEAS in membrane vesicles from MRP4-expressing Sf9 cells |
Biochem J 371: 361-7 (2003)
Article DOI: 10.1042/BJ20021886
BindingDB Entry DOI: 10.7270/Q2GH9K68 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13807
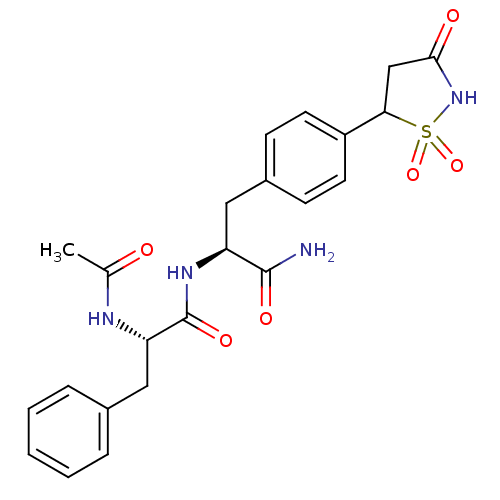
((2S)-N-[(1S)-1-carbamoyl-2-[4-(1,1,3-trioxo-1,2-th...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)C1CC(=O)NS1(=O)=O)C(N)=O |r| Show InChI InChI=1S/C23H26N4O6S/c1-14(28)25-19(12-15-5-3-2-4-6-15)23(31)26-18(22(24)30)11-16-7-9-17(10-8-16)20-13-21(29)27-34(20,32)33/h2-10,18-20H,11-13H2,1H3,(H2,24,30)(H,25,28)(H,26,31)(H,27,29)/t18-,19-,20?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation
| Assay Description
The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... |
J Biol Chem 281: 32784-95 (2006)
Article DOI: 10.1074/jbc.M606873200
BindingDB Entry DOI: 10.7270/Q2GX48SD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM185354

(US9156831, 5)Show SMILES C[C@H](O)CC(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(32)10-15(33)31-8-6-13(7-9-31)18-26-19(30(5)29-18)14-11-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h11-13,32H,6-10H2,1-5H3,(H2,23,24)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | 37 |
AstraZeneca AB
US Patent
| Assay Description
This assay was used to measure PI3K-alpha inhibition in cells. BT474 cells (human breast ductal carcinoma, ATCC HTB-20) were seeded into black 384 we... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM185353

(US9156831, 4)Show SMILES C[C@@H](O)CC(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(32)10-15(33)31-8-6-13(7-9-31)18-26-19(30(5)29-18)14-11-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h11-13,32H,6-10H2,1-5H3,(H2,23,24)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | 37 |
AstraZeneca AB
US Patent
| Assay Description
This assay was used to measure PI3K-alpha inhibition in cells. BT474 cells (human breast ductal carcinoma, ATCC HTB-20) were seeded into black 384 we... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM185358

(US9156831, 9)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CC(O)=O Show InChI InChI=1S/C22H29N9O4/c1-5-31-19(26-18(29-31)12-6-8-30(9-7-12)14(32)10-15(33)34)13-11-24-17(23)16(25-13)20-27-28-21(35-20)22(2,3)4/h11-12H,5-10H2,1-4H3,(H2,23,24)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | 37 |
AstraZeneca AB
US Patent
| Assay Description
This assay was used to measure PI3K-alpha inhibition in cells. BT474 cells (human breast ductal carcinoma, ATCC HTB-20) were seeded into black 384 we... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM185361

(US9156831, 1)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CCO Show InChI InChI=1S/C21H29N9O3/c1-21(2,3)20-27-26-19(33-20)15-16(22)23-11-13(24-15)18-25-17(28-29(18)4)12-5-8-30(9-6-12)14(32)7-10-31/h11-12,31H,5-10H2,1-4H3,(H2,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | 37 |
AstraZeneca AB
US Patent
| Assay Description
This assay was used to measure PI3K-alpha inhibition in cells. BT474 cells (human breast ductal carcinoma, ATCC HTB-20) were seeded into black 384 we... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM185356

(US9156831, 7)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)C(C)(C)O Show InChI InChI=1S/C22H31N9O3/c1-21(2,3)19-28-27-18(34-19)14-15(23)24-11-13(25-14)17-26-16(29-30(17)6)12-7-9-31(10-8-12)20(32)22(4,5)33/h11-12,33H,7-10H2,1-6H3,(H2,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | 37 |
AstraZeneca AB
US Patent
| Assay Description
This assay was used to measure PI3K-alpha inhibition in cells. BT474 cells (human breast ductal carcinoma, ATCC HTB-20) were seeded into black 384 we... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM185355

(US9156831, 6)Show SMILES C[C@H](CO)C(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(11-32)20(33)31-8-6-13(7-9-31)17-26-18(30(5)29-17)14-10-24-16(23)15(25-14)19-27-28-21(34-19)22(2,3)4/h10,12-13,32H,6-9,11H2,1-5H3,(H2,23,24)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | 37 |
AstraZeneca AB
US Patent
| Assay Description
This assay was used to measure PI3K-alpha inhibition in cells. BT474 cells (human breast ductal carcinoma, ATCC HTB-20) were seeded into black 384 we... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM185362

(US9156831, 3)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CCO Show InChI InChI=1S/C22H31N9O3/c1-5-31-19(26-18(29-31)13-6-9-30(10-7-13)15(33)8-11-32)14-12-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h12-13,32H,5-11H2,1-4H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM185357

(US9156831, 8)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CC(O)=O Show InChI InChI=1S/C21H27N9O4/c1-21(2,3)20-27-26-19(34-20)15-16(22)23-10-12(24-15)18-25-17(28-29(18)4)11-5-7-30(8-6-11)13(31)9-14(32)33/h10-11H,5-9H2,1-4H3,(H2,22,23)(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | 37 |
AstraZeneca AB
US Patent
| Assay Description
This assay was used to measure PI3K-alpha inhibition in cells. BT474 cells (human breast ductal carcinoma, ATCC HTB-20) were seeded into black 384 we... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM185358

(US9156831, 9)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CC(O)=O Show InChI InChI=1S/C22H29N9O4/c1-5-31-19(26-18(29-31)12-6-8-30(9-7-12)14(32)10-15(33)34)13-11-24-17(23)16(25-13)20-27-28-21(35-20)22(2,3)4/h11-12H,5-10H2,1-4H3,(H2,23,24)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13806
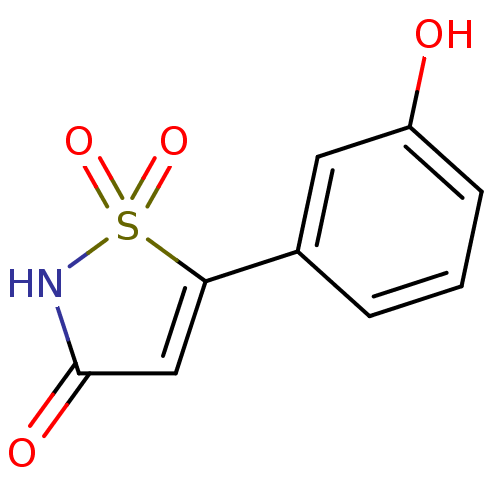
(5-(3-hydroxyphenyl)-2,3-dihydro-1,2-thiazole-1,1,3...)Show InChI InChI=1S/C9H7NO4S/c11-7-3-1-2-6(4-7)8-5-9(12)10-15(8,13)14/h1-5,11H,(H,10,12) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation
| Assay Description
The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... |
J Biol Chem 281: 32784-95 (2006)
Article DOI: 10.1074/jbc.M606873200
BindingDB Entry DOI: 10.7270/Q2GX48SD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM185357

(US9156831, 8)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CC(O)=O Show InChI InChI=1S/C21H27N9O4/c1-21(2,3)20-27-26-19(34-20)15-16(22)23-10-12(24-15)18-25-17(28-29(18)4)11-5-7-30(8-6-11)13(31)9-14(32)33/h10-11H,5-9H2,1-4H3,(H2,22,23)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13472

(({4-[(2S)-2-carbamoyl-2-[(2S)-2-acetamido-3-phenyl...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C21H24F2N3O6P/c1-13(27)25-18(12-14-5-3-2-4-6-14)20(29)26-17(19(24)28)11-15-7-9-16(10-8-15)21(22,23)33(30,31)32/h2-10,17-18H,11-12H2,1H3,(H2,24,28)(H,25,27)(H,26,29)(H2,30,31,32)/t17-,18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation
| Assay Description
The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... |
J Biol Chem 281: 32784-95 (2006)
Article DOI: 10.1074/jbc.M606873200
BindingDB Entry DOI: 10.7270/Q2GX48SD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13472

(({4-[(2S)-2-carbamoyl-2-[(2S)-2-acetamido-3-phenyl...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C21H24F2N3O6P/c1-13(27)25-18(12-14-5-3-2-4-6-14)20(29)26-17(19(24)28)11-15-7-9-16(10-8-15)21(22,23)33(30,31)32/h2-10,17-18H,11-12H2,1H3,(H2,24,28)(H,25,27)(H,26,29)(H2,30,31,32)/t17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein-tyrosine phosphatase 1B by PNPP enzyme assay |
J Med Chem 48: 6544-8 (2005)
Article DOI: 10.1021/jm0504555
BindingDB Entry DOI: 10.7270/Q2805252 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ATP-binding cassette sub-family C member 4
(Homo sapiens (Human)) | BDBM50270011

(estradiol disulfate)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS([O-])(=O)=O)ccc34)[C@@H]1CC[C@@H]2OS([O-])(=O)=O |r| Show InChI InChI=1S/C18H24O8S2/c1-18-9-8-14-13-5-3-12(25-27(19,20)21)10-11(13)2-4-15(14)16(18)6-7-17(18)26-28(22,23)24/h3,5,10,14-17H,2,4,6-9H2,1H3,(H,19,20,21)(H,22,23,24)/p-2/t14-,15-,16+,17+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Cancer Institute
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of E217betaG uptake in membrane vesicles from MRP4-expressing Sf9 cells |
Biochem J 371: 361-7 (2003)
Article DOI: 10.1042/BJ20021886
BindingDB Entry DOI: 10.7270/Q2GH9K68 |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 4
(Homo sapiens (Human)) | BDBM23620
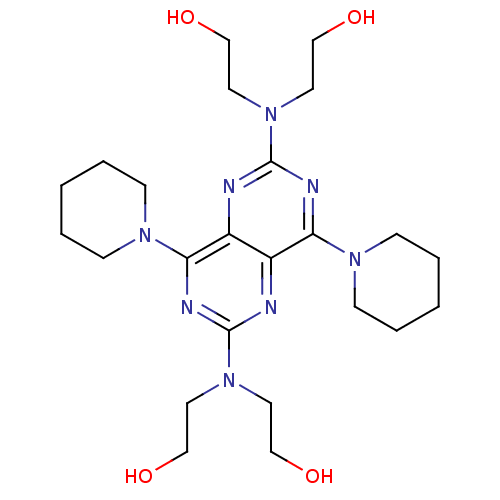
(2-({6-[bis(2-hydroxyethyl)amino]-4,8-bis(piperidin...)Show SMILES OCCN(CCO)c1nc(N2CCCCC2)c2nc(nc(N3CCCCC3)c2n1)N(CCO)CCO Show InChI InChI=1S/C24H40N8O4/c33-15-11-31(12-16-34)23-26-20-19(21(27-23)29-7-3-1-4-8-29)25-24(32(13-17-35)14-18-36)28-22(20)30-9-5-2-6-10-30/h33-36H,1-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Cancer Institute
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of 9-(2-phosphonomethoxyethyl)adenine(PMEA) efflux (PMEA: 1 uM) in MRP4-expressing HEK293 cells |
Mol Pharmacol 63: 1094-103 (2003)
BindingDB Entry DOI: 10.7270/Q2KH0PMD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM185361

(US9156831, 1)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CCO Show InChI InChI=1S/C21H29N9O3/c1-21(2,3)20-27-26-19(33-20)15-16(22)23-11-13(24-15)18-25-17(28-29(18)4)12-5-8-30(9-6-12)14(32)7-10-31/h11-12,31H,5-10H2,1-4H3,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM185353

(US9156831, 4)Show SMILES C[C@@H](O)CC(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(32)10-15(33)31-8-6-13(7-9-31)18-26-19(30(5)29-18)14-11-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h11-13,32H,6-10H2,1-5H3,(H2,23,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13811

((2S)-N-[(1S)-1-carbamoyl-2-[4-(1,1,3-trioxo-2,3-di...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)C1=CC(=O)NS1(=O)=O)C(N)=O |r,t:25| Show InChI InChI=1S/C23H24N4O6S/c1-14(28)25-19(12-15-5-3-2-4-6-15)23(31)26-18(22(24)30)11-16-7-9-17(10-8-16)20-13-21(29)27-34(20,32)33/h2-10,13,18-19H,11-12H2,1H3,(H2,24,30)(H,25,28)(H,26,31)(H,27,29)/t18-,19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Incyte Corporation
| Assay Description
The activity of PTP1B enzyme was assayed with 4-nitrophenyl phosphate (pNPP) as substrate. Rate of formation of the phenolate ion was monitored at 41... |
J Biol Chem 281: 32784-95 (2006)
Article DOI: 10.1074/jbc.M606873200
BindingDB Entry DOI: 10.7270/Q2GX48SD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13811

((2S)-N-[(1S)-1-carbamoyl-2-[4-(1,1,3-trioxo-2,3-di...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)C1=CC(=O)NS1(=O)=O)C(N)=O |r,t:25| Show InChI InChI=1S/C23H24N4O6S/c1-14(28)25-19(12-15-5-3-2-4-6-15)23(31)26-18(22(24)30)11-16-7-9-17(10-8-16)20-13-21(29)27-34(20,32)33/h2-10,13,18-19H,11-12H2,1H3,(H2,24,30)(H,25,28)(H,26,31)(H,27,29)/t18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein-tyrosine phosphatase 1B by PNPP enzyme assay |
J Med Chem 48: 6544-8 (2005)
Article DOI: 10.1021/jm0504555
BindingDB Entry DOI: 10.7270/Q2805252 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM185354

(US9156831, 5)Show SMILES C[C@H](O)CC(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(32)10-15(33)31-8-6-13(7-9-31)18-26-19(30(5)29-18)14-11-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h11-13,32H,6-10H2,1-5H3,(H2,23,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM185355

(US9156831, 6)Show SMILES C[C@H](CO)C(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(11-32)20(33)31-8-6-13(7-9-31)17-26-18(30(5)29-17)14-10-24-16(23)15(25-14)19-27-28-21(34-19)22(2,3)4/h10,12-13,32H,6-9,11H2,1-5H3,(H2,23,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM185356

(US9156831, 7)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)C(C)(C)O Show InChI InChI=1S/C22H31N9O3/c1-21(2,3)19-28-27-18(34-19)14-15(23)24-11-13(25-14)17-26-16(29-30(17)6)12-7-9-31(10-8-12)20(32)22(4,5)33/h11-12,33H,7-10H2,1-6H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.26E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 4
(Homo sapiens (Human)) | BDBM50375575
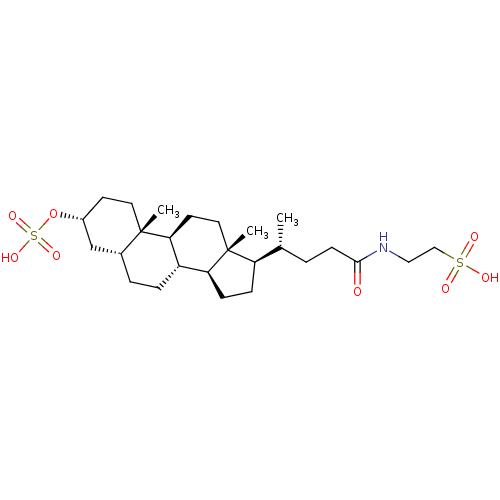
(CHEMBL270493)Show SMILES C[C@H](CCC(=O)NCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@@H](CC[C@]4(C)[C@H]3CC[C@]12C)OS(O)(=O)=O Show InChI InChI=1S/C26H45NO8S2/c1-17(4-9-24(28)27-14-15-36(29,30)31)21-7-8-22-20-6-5-18-16-19(35-37(32,33)34)10-12-25(18,2)23(20)11-13-26(21,22)3/h17-23H,4-16H2,1-3H3,(H,27,28)(H,29,30,31)(H,32,33,34)/t17-,18-,19-,20+,21-,22+,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Cancer Institute
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of E217betaG uptake in membrane vesicles from MRP4-expressing HEK-293 cells |
Biochem J 371: 361-7 (2003)
Article DOI: 10.1042/BJ20021886
BindingDB Entry DOI: 10.7270/Q2GH9K68 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data