

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | US Patent | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222959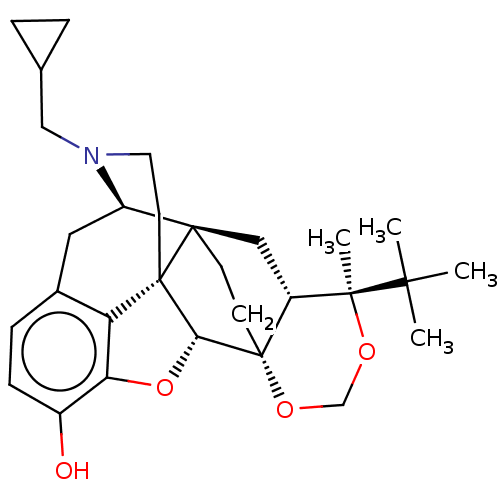 (US9315514, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222960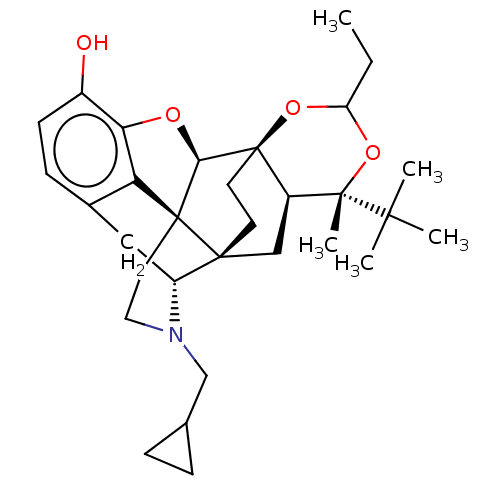 (US9315514, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.190 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | US Patent | 0.300 | -58.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Rhodes Technologies US Patent | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222960 (US9315514, 4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.350 | -58.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Rhodes Technologies US Patent | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222963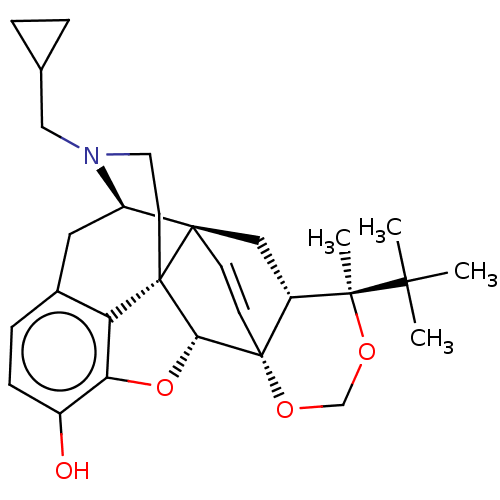 (US9315514, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.440 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222959 (US9315514, 3) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.450 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Rhodes Technologies US Patent | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222961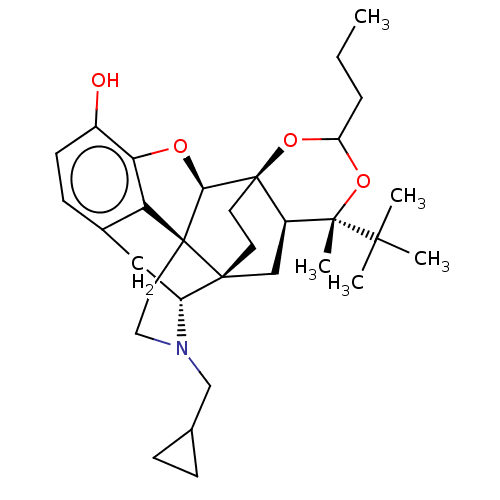 (US9315514, 5) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.540 | -57.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Rhodes Technologies US Patent | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222961 (US9315514, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.720 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222962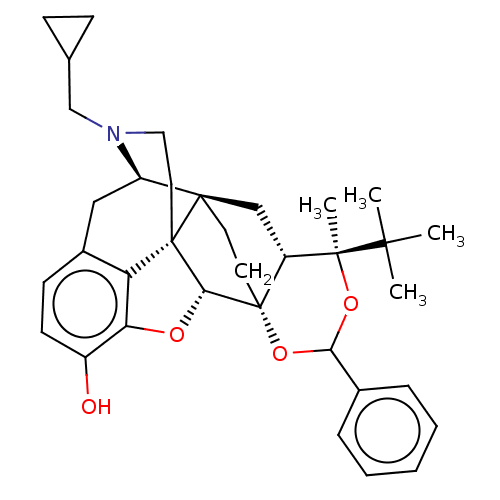 (US9315514, 6) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.26 | -53.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Rhodes Technologies US Patent | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222962 (US9315514, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.61 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222965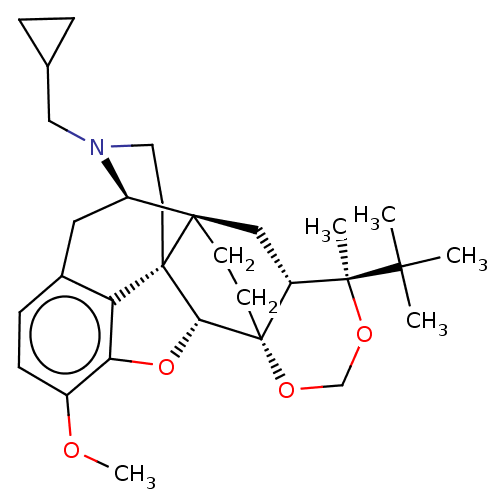 (US9315514, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.22 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222964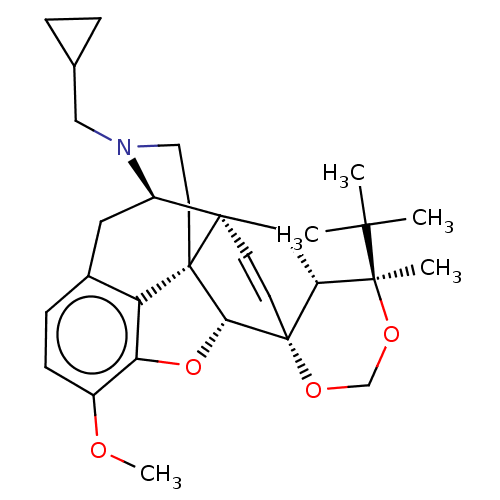 (US9315514, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30.1 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222966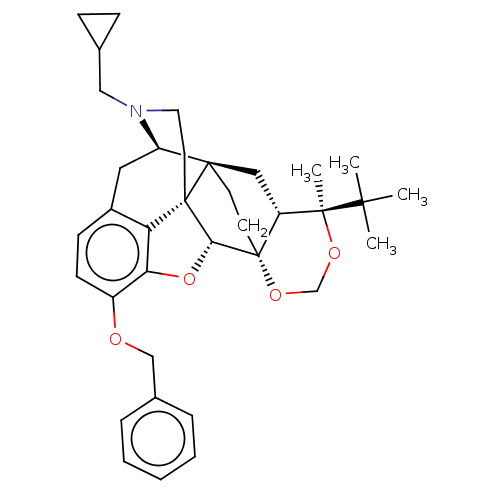 (US9315514, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 757 | -34.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 0.450 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description [35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared in-house from a cell line expressing recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222966 (US9315514, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Functional [35S]GTPγS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding f... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 0.560 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Functional [35S]GTPγS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding f... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222959 (US9315514, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 22.7 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Functional [35S]GTPγS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding f... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222960 (US9315514, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 4.42 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Functional [35S]GTPγS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding f... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222961 (US9315514, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 9.48 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Functional [35S]GTPγS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding f... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222962 (US9315514, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >20 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Functional [35S]GTPγS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding f... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222963 (US9315514, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7.76 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Functional [35S]GTPγS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding f... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222964 (US9315514, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 593 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Functional [35S]GTPγS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding f... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222965 (US9315514, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 855 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Functional [35S]GTPγS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding f... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222966 (US9315514, 7) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 950 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description [35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared in-house from a cell line expressing recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222965 (US9315514, 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 85.4 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description [35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared in-house from a cell line expressing recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222964 (US9315514, 12) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 460 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description [35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared in-house from a cell line expressing recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222963 (US9315514, 11) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.56 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description [35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared in-house from a cell line expressing recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222962 (US9315514, 6) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 5.35 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description [35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared in-house from a cell line expressing recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222959 (US9315514, 3) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.860 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description [35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared in-house from a cell line expressing recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222960 (US9315514, 4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description [35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared in-house from a cell line expressing recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222961 (US9315514, 5) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.54 | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description [35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared in-house from a cell line expressing recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||