

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM176047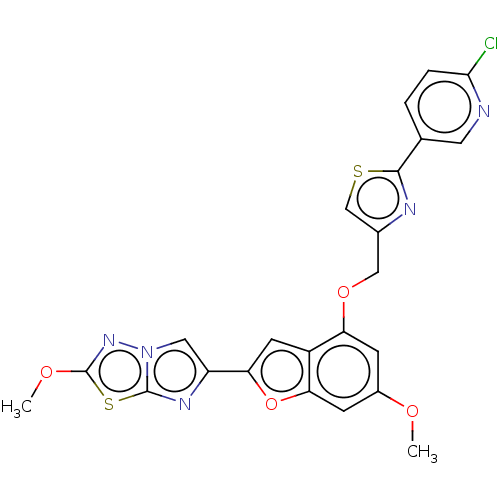 (US10047103, 80 | US9688695, 80) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176041 (US10047103, 74 | US9688695, 74) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176255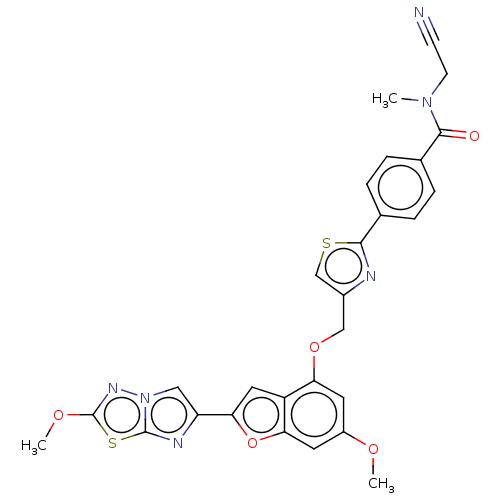 (US10047103, 288 | US9688695, 288) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176015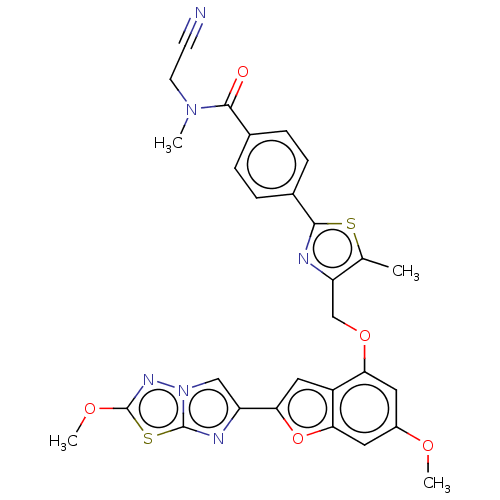 (US10047103, 48 | US9688695, 48) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM175995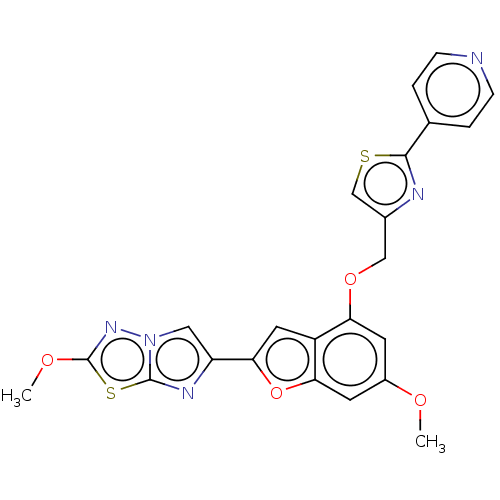 (US10047103, 75 | US9605024, Example 28 | US9688695...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176048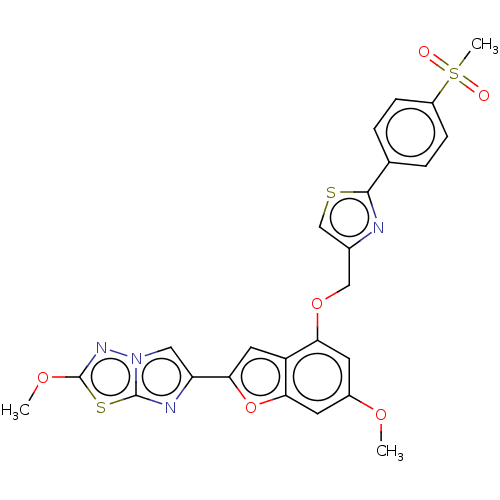 (US10047103, 81 | US9688695, 81) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176268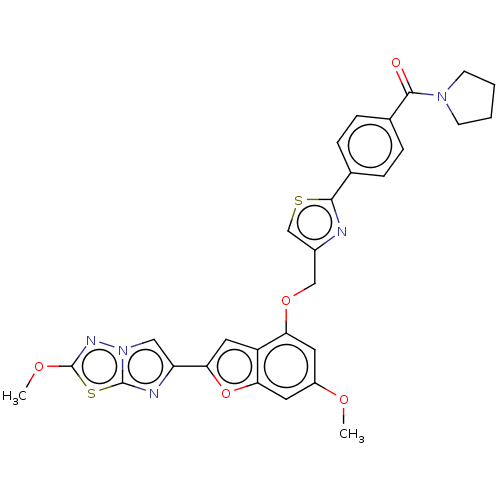 (US10047103, 301 | US9688695, 301) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176044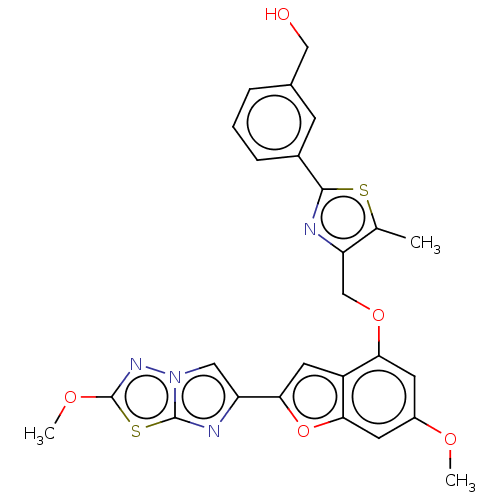 (US10047103, 77 | US9688695, 77) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176040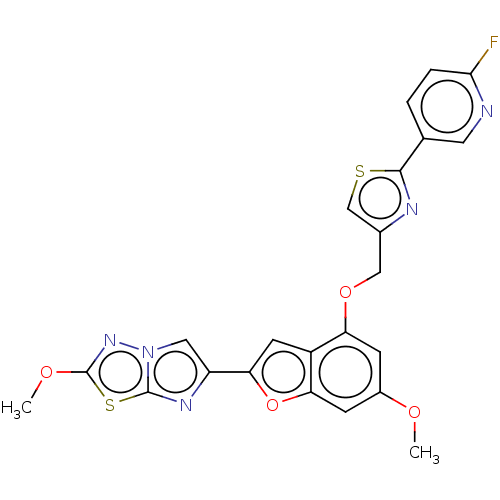 (US10047103, 73 | US9688695, 73) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176093 (US10047103, 126 | US9688695, 126 | US9688695, 127) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176272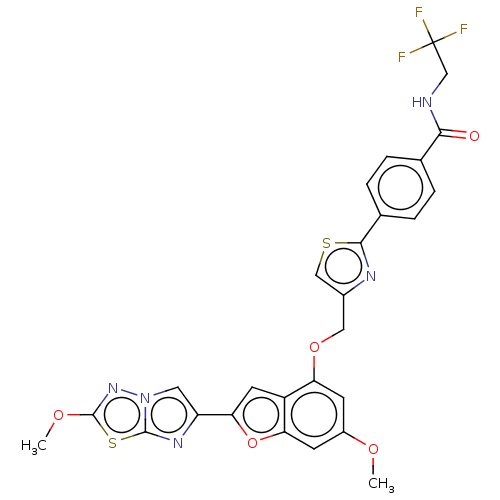 (US10047103, 305 | US9688695, 305) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176278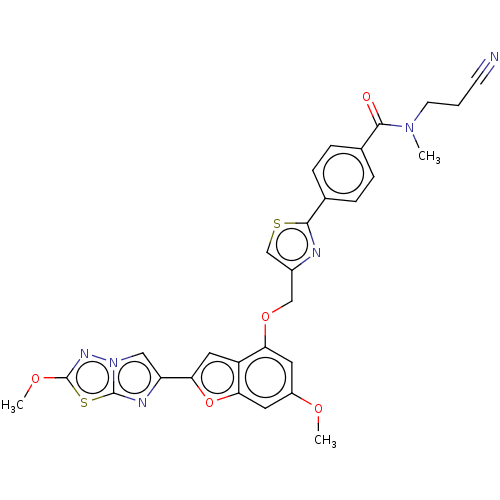 (US10047103, 311 | US9688695, 311) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176011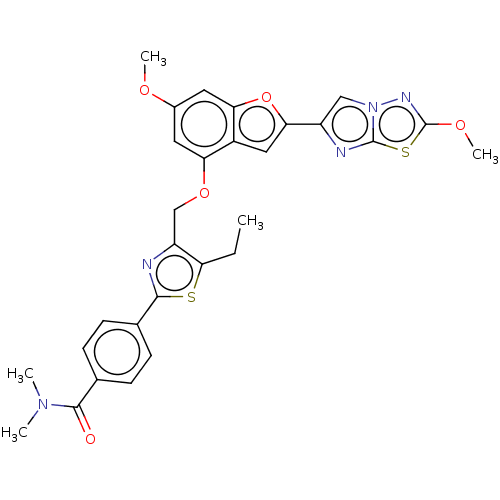 (US10047103, 44 | US9688695, 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176003 (US10047103, 36 | US9688695, 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176006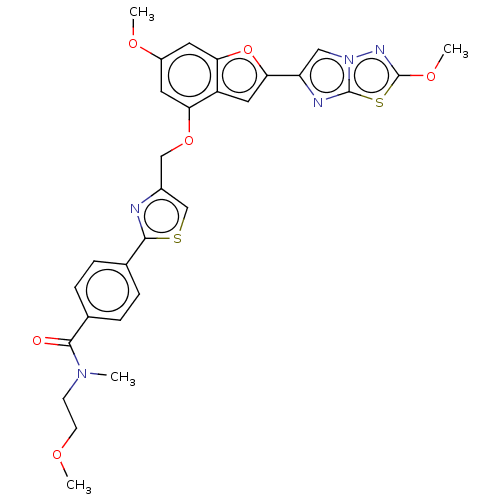 (US10047103, 39 | US9688695, 39) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176034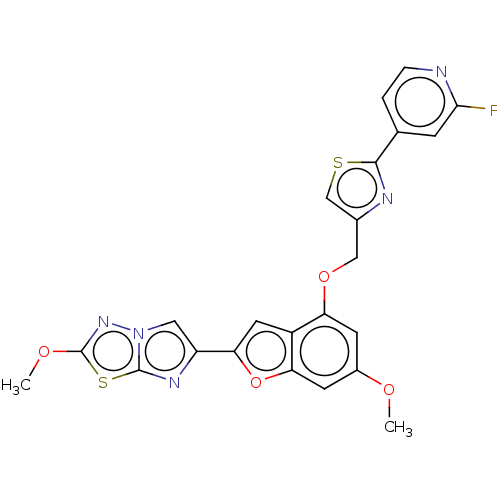 (US10047103, 67 | US9688695, 67) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM175995 (US10047103, 75 | US9605024, Example 28 | US9688695...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM175985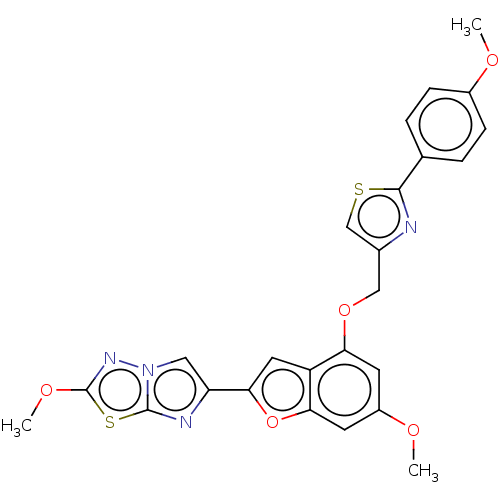 (US10047103, 18 | US9605024, Example 18 | US9688695...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM175970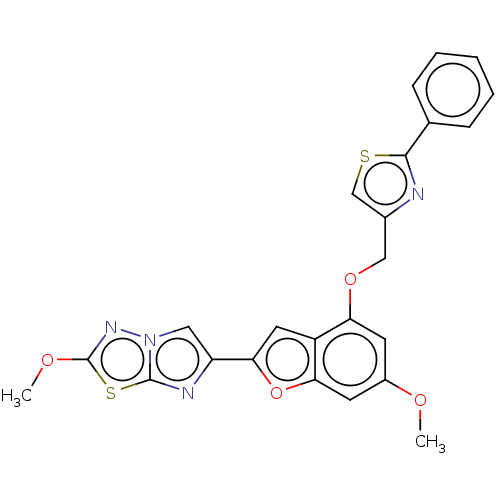 (US10047103, 3 | US9605024, Example 3 | US9688695, ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176092 (US10047103, 125 | US9688695, 125) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176061 (US10047103, 94 | US9688695, 94) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50184633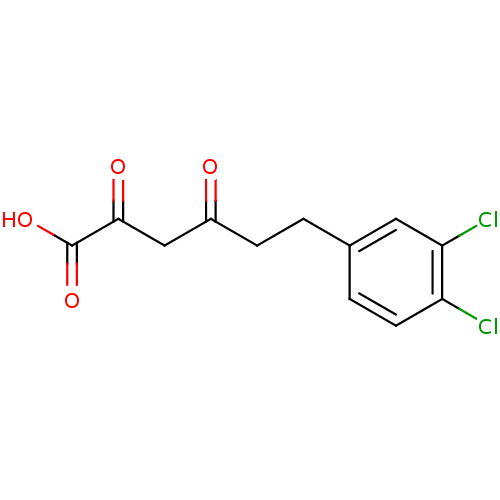 (6-(3,4-dichlorophenyl)-2-hydroxy-4-oxohex-2-enoic ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of HIV integrase | Bioorg Med Chem Lett 16: 2920-4 (2006) Article DOI: 10.1016/j.bmcl.2006.03.010 BindingDB Entry DOI: 10.7270/Q2BV7HDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM23399 (4-{1-[(4-fluorophenyl)methyl]-1H-pyrrol-2-yl}-2,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of HIV integrase | Bioorg Med Chem Lett 16: 2920-4 (2006) Article DOI: 10.1016/j.bmcl.2006.03.010 BindingDB Entry DOI: 10.7270/Q2BV7HDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176176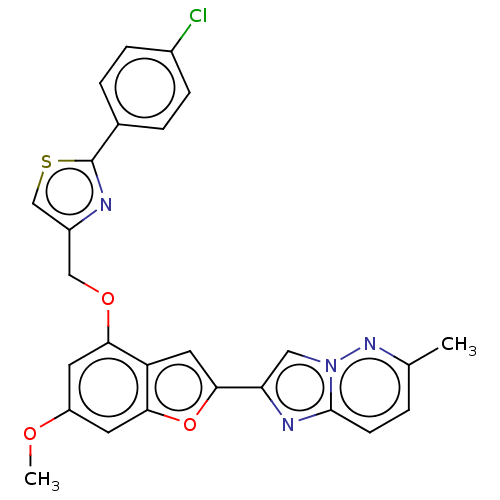 (US10047103, 209 | US9688695, 209) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176023 (US10047103, 56 | US9688695, 56) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176104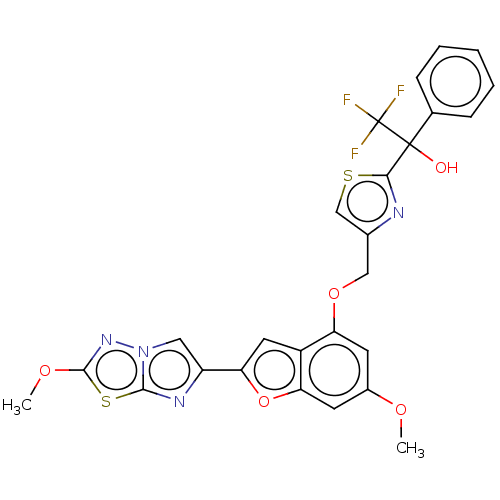 (US10047103, 137 | US9688695, 137) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176108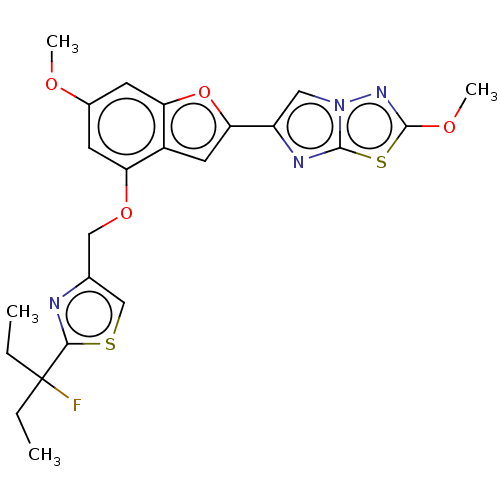 (US10047103, 141 | US9688695, 141) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176070 (US10047103, 103 | US9688695, 103) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176186 (US10047103, 219 | US9688695, 219) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176085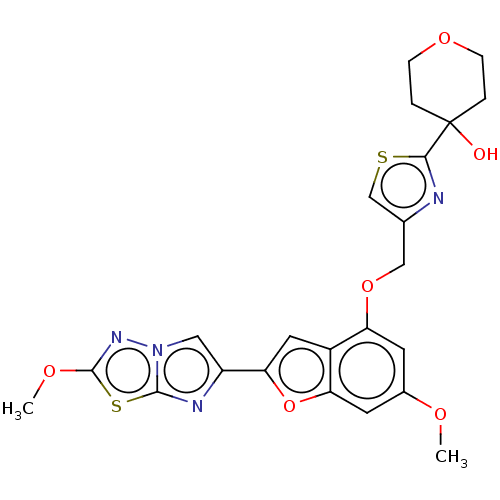 (US10047103, 118 | US9688695, 118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176118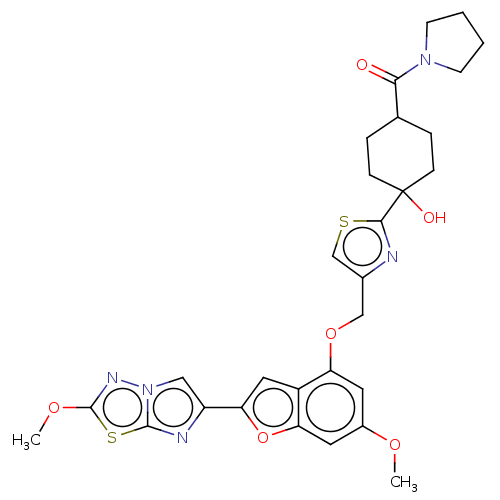 (US10047103, 151 | US9688695, 151) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176106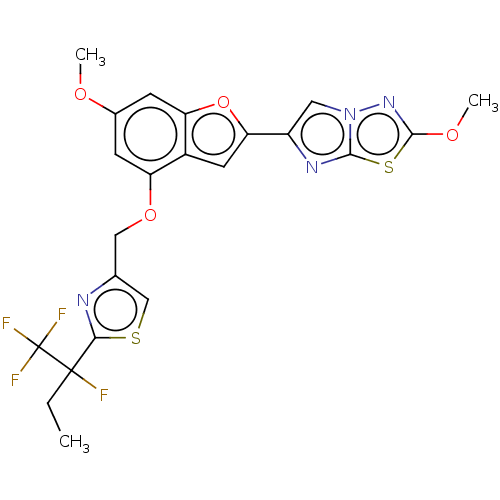 (US10047103, 139 | US9688695, 139) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176128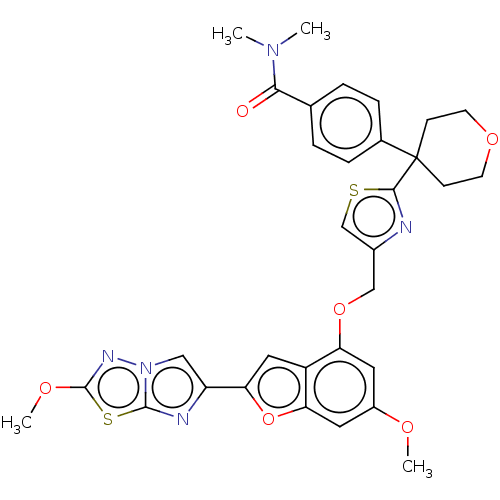 (US10047103, 161 | US9688695, 161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176088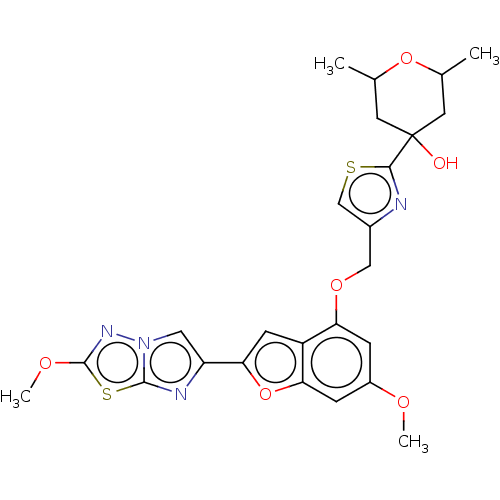 (US10047103, 121 | US9688695, 121) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176060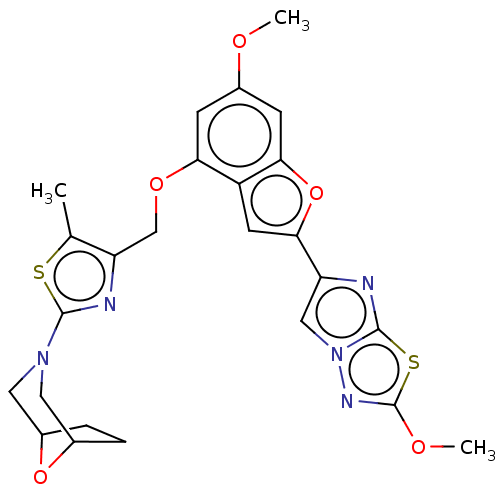 (US10047103, 93 | US9688695, 93) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176159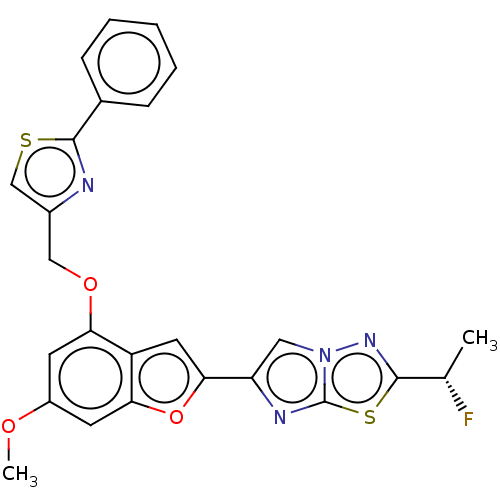 (US10047103, 192 | US9688695, 192) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176119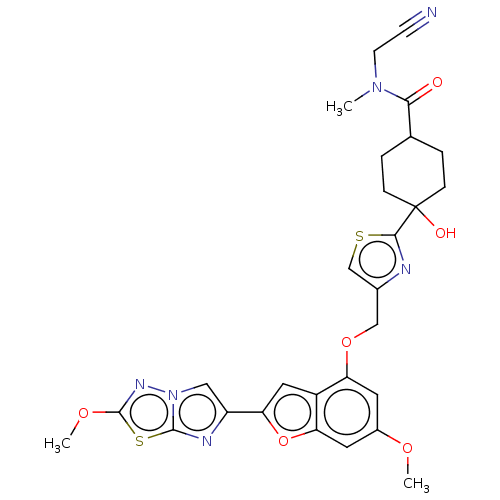 (US10047103, 152 | US9688695, 152) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176082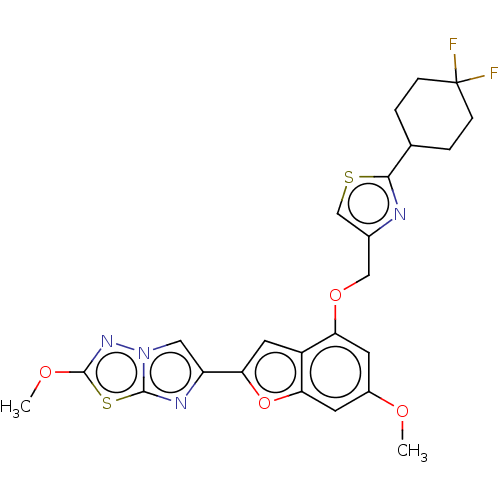 (US10047103, 115 | US9688695, 115) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176112 (US10047103, 145 | US9688695, 145) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176000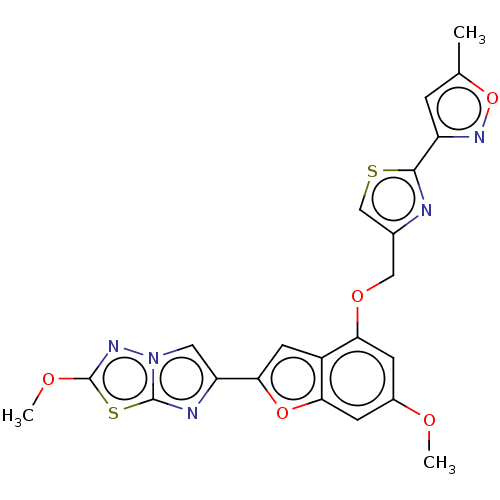 (US10047103, 33 | US9605024, Example 33 | US9688695...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM175969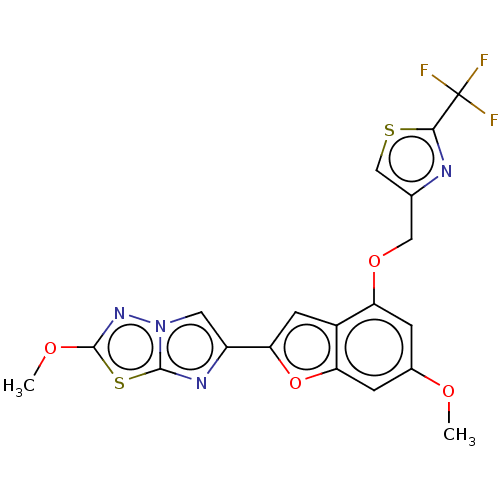 (US10047103, 2 | US9605024, Example 2 | US9688695, ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM107693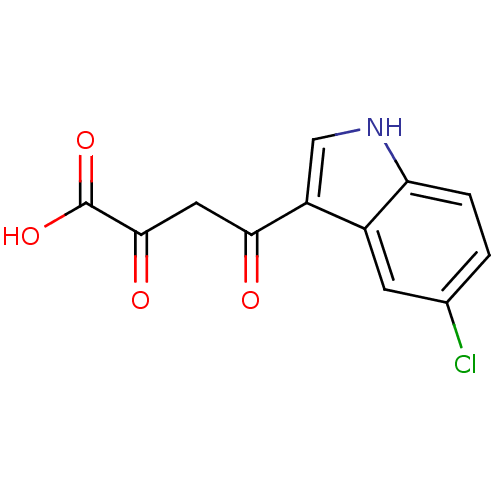 ((2Z)-4-(5-chloro-1H-indol-3-yl)-2-hydroxy-4-oxobut...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of HIV integrase | Bioorg Med Chem Lett 16: 2920-4 (2006) Article DOI: 10.1016/j.bmcl.2006.03.010 BindingDB Entry DOI: 10.7270/Q2BV7HDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061136 ((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50369315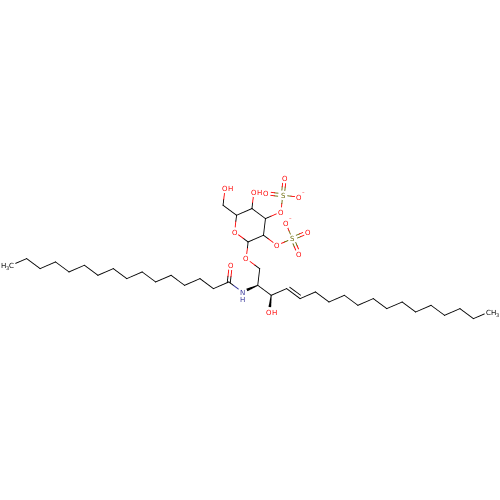 (CHEMBL1627019) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061124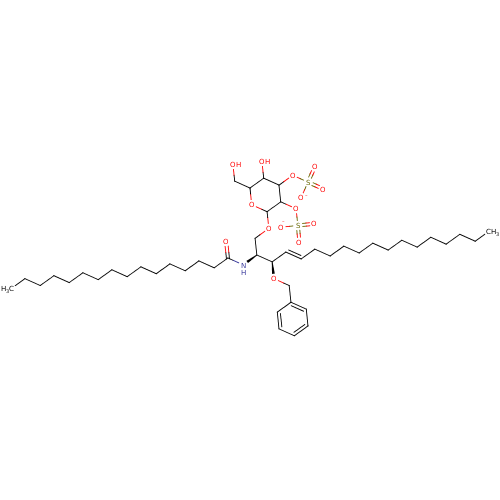 ((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061130 ((2S,3R,4E)-3-(Benzoyloxy)-2-(hexadecanoylamino)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176168 (US10047103, 201 | US9688695, 201) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50184626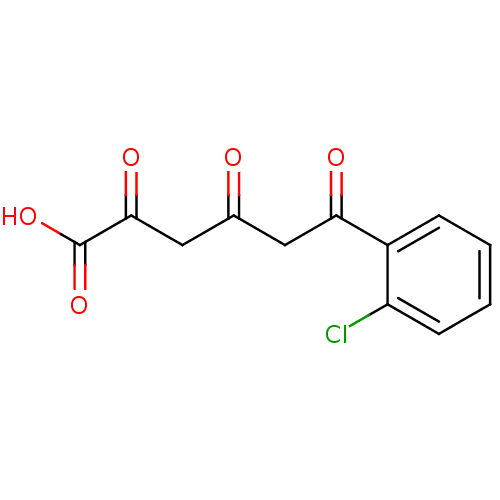 (6-(2-chlorophenyl)-2,4,6-trioxohexanoic acid | CHE...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of HIV integrase | Bioorg Med Chem Lett 16: 2920-4 (2006) Article DOI: 10.1016/j.bmcl.2006.03.010 BindingDB Entry DOI: 10.7270/Q2BV7HDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM23399 (4-{1-[(4-fluorophenyl)methyl]-1H-pyrrol-2-yl}-2,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase | Bioorg Med Chem Lett 16: 2920-4 (2006) Article DOI: 10.1016/j.bmcl.2006.03.010 BindingDB Entry DOI: 10.7270/Q2BV7HDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50061125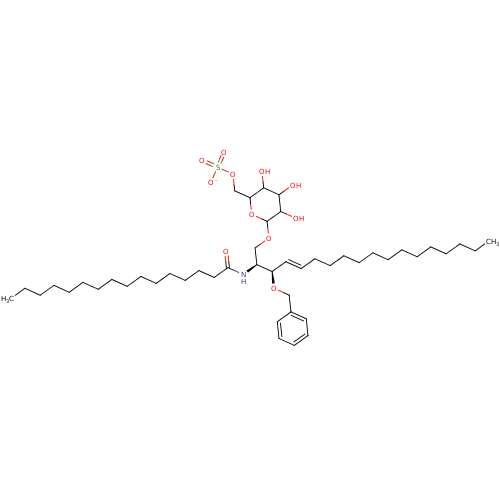 ((2S,3R,4E)-2-(hexadecanoylamino)-3-(benzoyloxy)-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Selectin P in a cell-free binding assay | J Med Chem 40: 3234-47 (1997) Article DOI: 10.1021/jm9606960 BindingDB Entry DOI: 10.7270/Q21Z454Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 426 total ) | Next | Last >> |