

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20000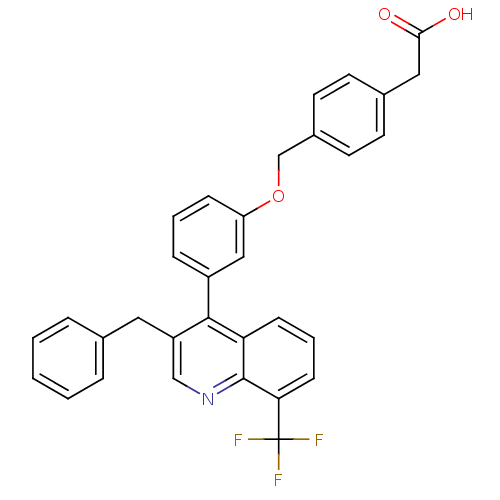 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | 90 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20001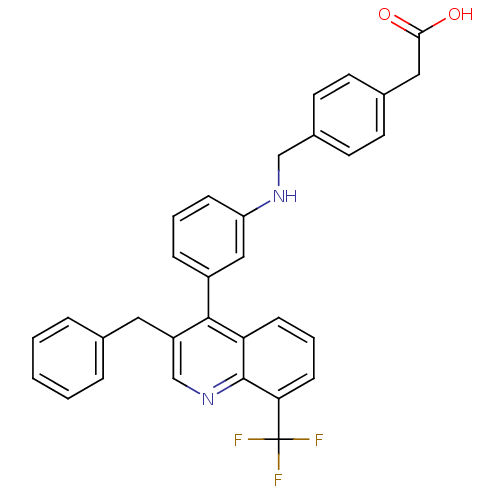 (2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | 90 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20001 (2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | 160 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 9 | n/a | 170 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26065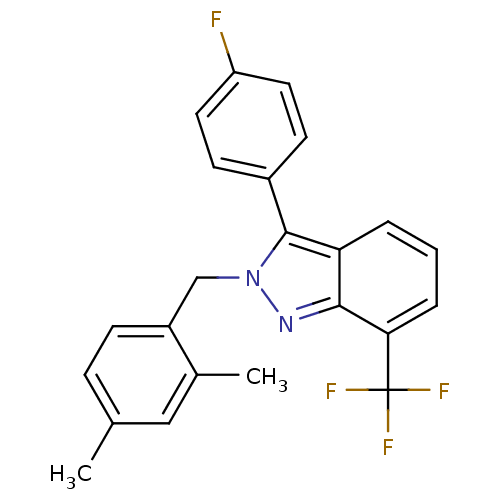 (2-[(2,4-dimethylphenyl)methyl]-3-(4-fluorophenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | 990 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20000 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | 240 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | 310 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 13 | n/a | 140 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26066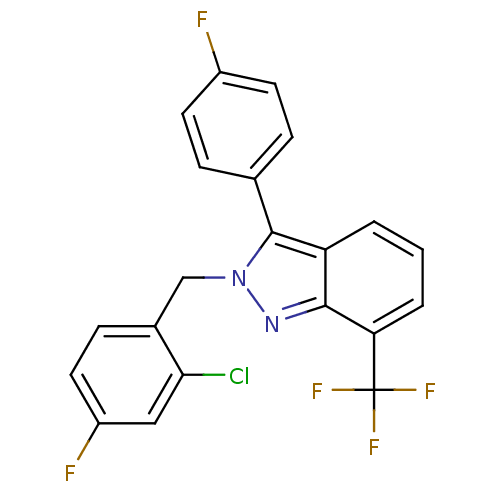 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | 3.67E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26072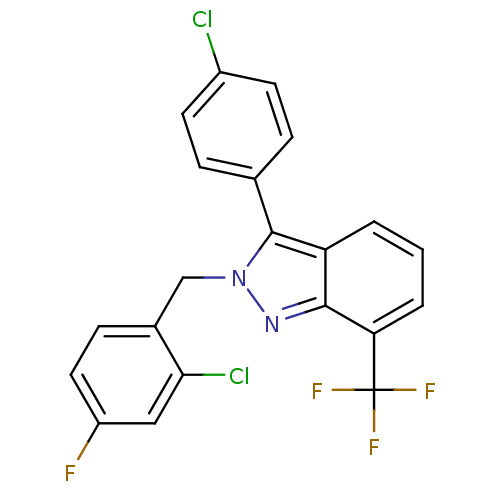 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-chlorophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | 1.12E+4 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26068 (2-[(2-chlorophenyl)methyl]-3-(4-fluorophenyl)-7-(t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | 2.89E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26067 (3-(4-fluorophenyl)-2-[(2-fluorophenyl)methyl]-7-(t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | 4.15E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26074 (2-benzyl-3-aryl-7-trifluoromethylindazole, 20 | 3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | 7.44E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26071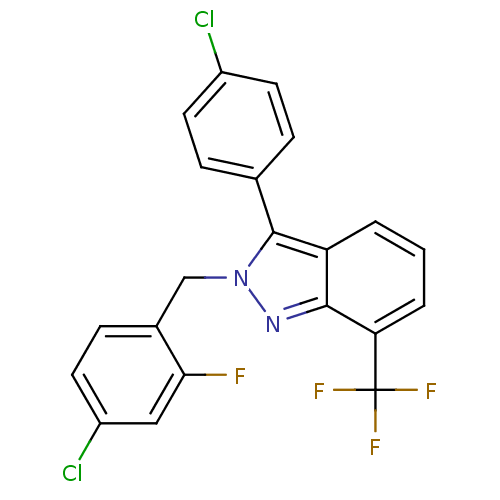 (2-[(4-chloro-2-fluorophenyl)methyl]-3-(4-chlorophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | 9.80E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26073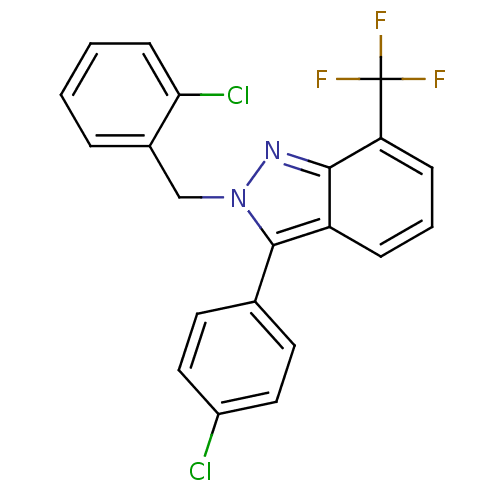 (2-benzyl-3-aryl-7-trifluoromethylindazole, 19 | 3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | 1.02E+4 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26064 (2-benzyl-3-aryl-7-trifluoromethylindazole, 5 | 2-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | 3.26E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26075 (2-benzyl-3-aryl-7-trifluoromethylindazole, 21 | 3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | 1.55E+4 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26069 (2-benzyl-3-aryl-7-trifluoromethylindazole, 15 | 3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | 5.72E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26070 (2-[(4-chlorophenyl)methyl]-3-(4-fluorophenyl)-7-(t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | 9.05E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26065 (2-[(2,4-dimethylphenyl)methyl]-3-(4-fluorophenyl)-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | 1.81E+3 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | 660 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26066 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 179 | n/a | 6.66E+3 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26067 (3-(4-fluorophenyl)-2-[(2-fluorophenyl)methyl]-7-(t...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 248 | n/a | 6.10E+3 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26072 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-chlorophe...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 252 | n/a | 1.44E+4 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26071 (2-[(4-chloro-2-fluorophenyl)methyl]-3-(4-chlorophe...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | 1.33E+4 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26064 (2-benzyl-3-aryl-7-trifluoromethylindazole, 5 | 2-b...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 279 | n/a | 4.27E+3 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26068 (2-[(2-chlorophenyl)methyl]-3-(4-fluorophenyl)-7-(t...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 297 | n/a | 5.02E+3 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26074 (2-benzyl-3-aryl-7-trifluoromethylindazole, 20 | 3-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 304 | n/a | 1.34E+4 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26069 (2-benzyl-3-aryl-7-trifluoromethylindazole, 15 | 3-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 317 | n/a | 8.40E+3 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26073 (2-benzyl-3-aryl-7-trifluoromethylindazole, 19 | 3-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 379 | n/a | 1.52E+4 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26070 (2-[(4-chlorophenyl)methyl]-3-(4-fluorophenyl)-7-(t...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 401 | n/a | 1.28E+4 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26075 (2-benzyl-3-aryl-7-trifluoromethylindazole, 21 | 3-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 465 | n/a | 1.58E+4 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26076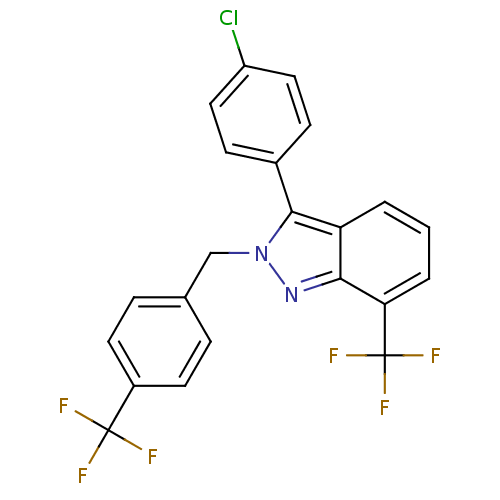 (2-benzyl-3-aryl-7-trifluoromethylindazole, 22 | 3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM26076 (2-benzyl-3-aryl-7-trifluoromethylindazole, 22 | 3-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||