

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254430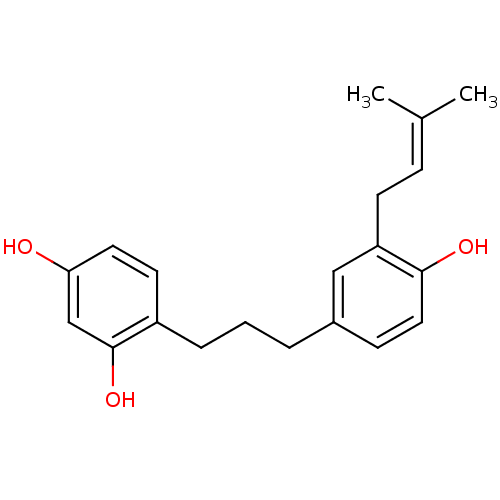 (CHEMBL468906 | broussonin C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 48.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase by kinetic based assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50251001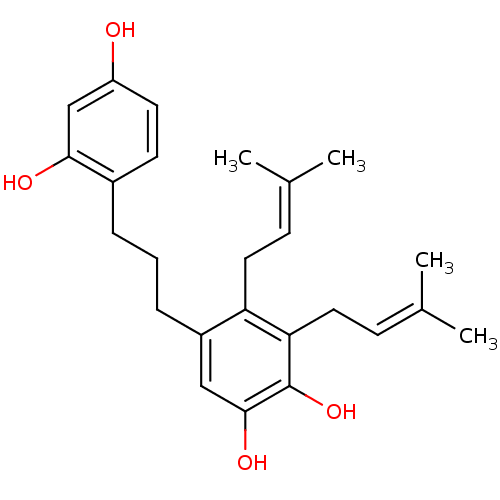 (CHEMBL457677 | Kazinol F) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase by kinetic based assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254430 (CHEMBL468906 | broussonin C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254430 (CHEMBL468906 | broussonin C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50251001 (CHEMBL457677 | Kazinol F) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50251001 (CHEMBL457677 | Kazinol F) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254431 (5'-(2-methylbut-3-en-2-yl)-6''-(3-methylbut-2-enyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase by kinetic based assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254431 (5'-(2-methylbut-3-en-2-yl)-6''-(3-methylbut-2-enyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254429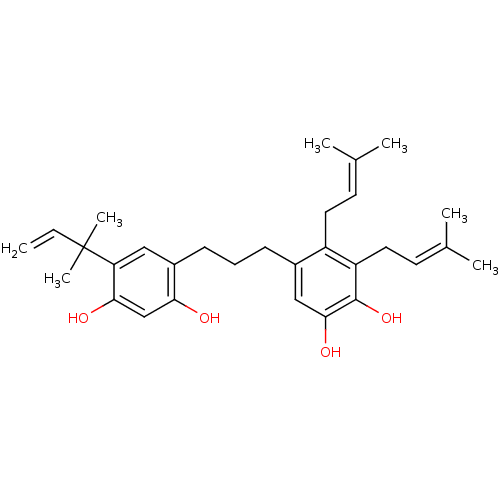 (CHEMBL466200 | kazinol C) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254429 (CHEMBL466200 | kazinol C) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254431 (5'-(2-methylbut-3-en-2-yl)-6''-(3-methylbut-2-enyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate by Lineweaver-Burk plot based kinetic assay | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50078850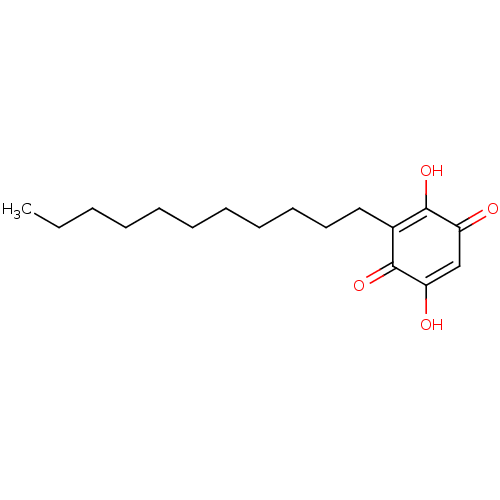 (CHEBI:4778 | Embelin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomes | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50366305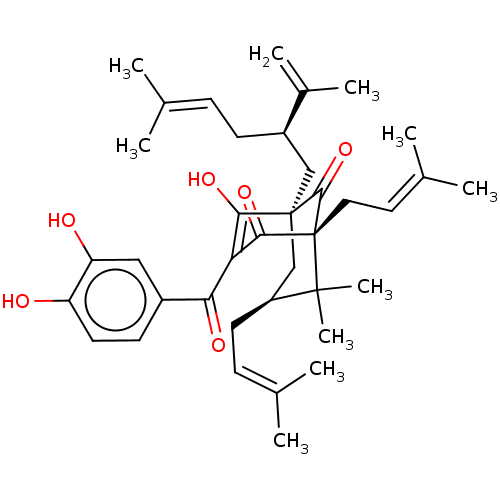 (CHEBI:70328 | CHEMBL445599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50109833 (Arzanol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50369678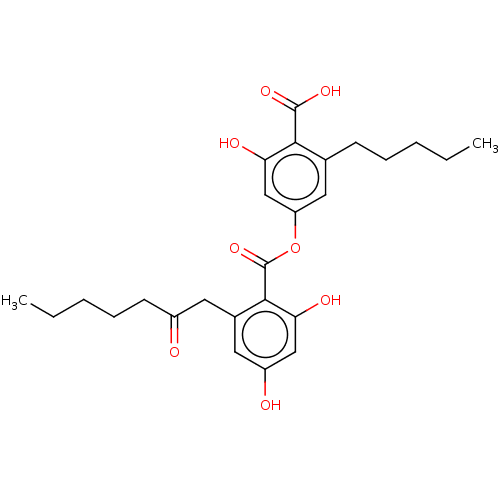 (CHEMBL3770253) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50369679 (CHEMBL2006643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254430 (CHEMBL468906 | broussonin C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254430 (CHEMBL468906 | broussonin C) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50251001 (CHEMBL457677 | Kazinol F) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50193079 (CHEBI:5834 | CHEMBL1237210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 (unknown origin) assessed as reduction in conversion of PGH to PGE2 | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50056919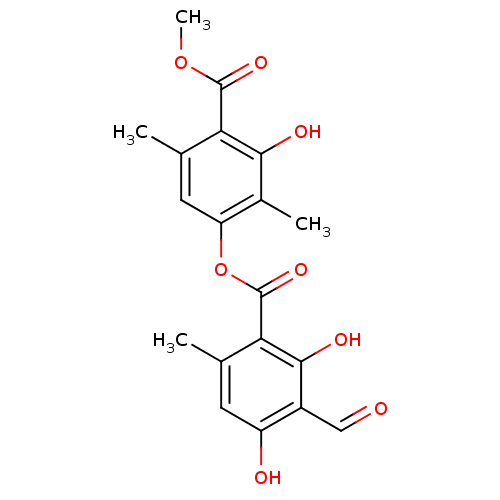 (3-hydroxy-4-(methoxycarbonyl)-2,5-dimethylphenyl 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50251001 (CHEMBL457677 | Kazinol F) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50070942 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50369681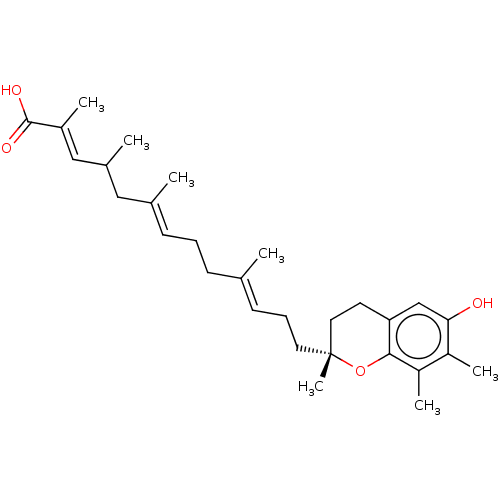 (CHEMBL4163039) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomes | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50369680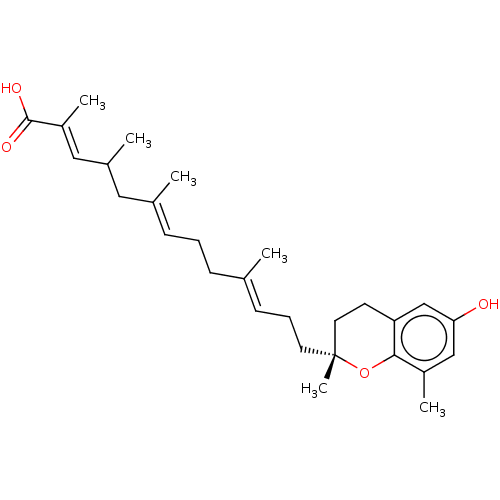 (CHEMBL4170957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50241262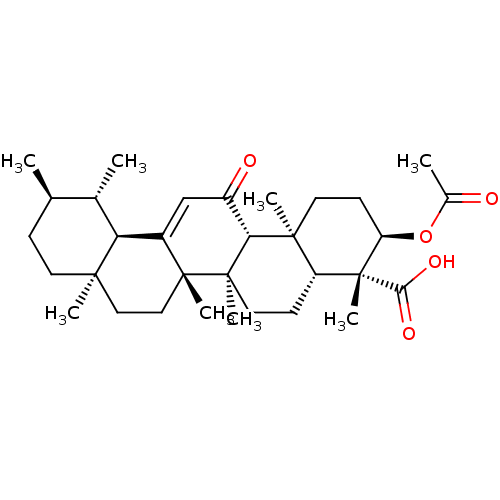 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bS)-3-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50371232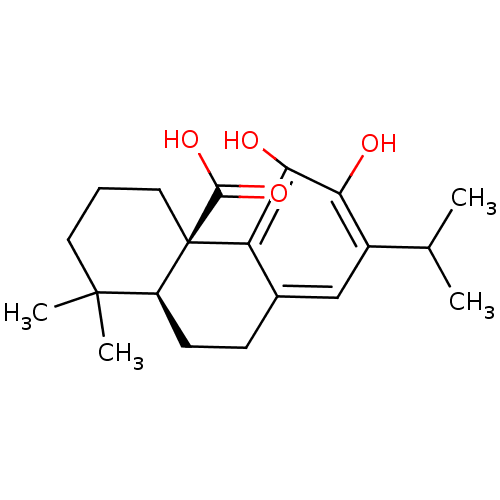 (CARNOSIC ACID) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50241260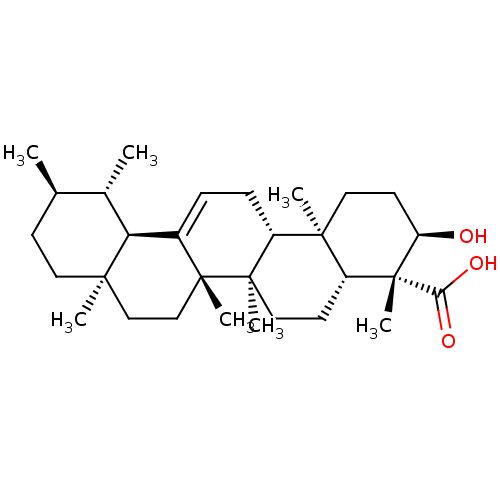 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50213683 (CHEMBL218693 | carnosol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate pretreated for 5 mins followed by substrate addition and measured after 5 mins by HPLC a... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50241261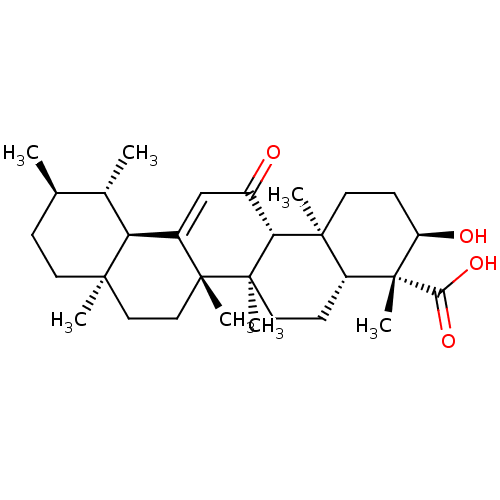 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bS)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254429 (CHEMBL466200 | kazinol C) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254431 (5'-(2-methylbut-3-en-2-yl)-6''-(3-methylbut-2-enyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254429 (CHEMBL466200 | kazinol C) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50254431 (5'-(2-methylbut-3-en-2-yl)-6''-(3-methylbut-2-enyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50253972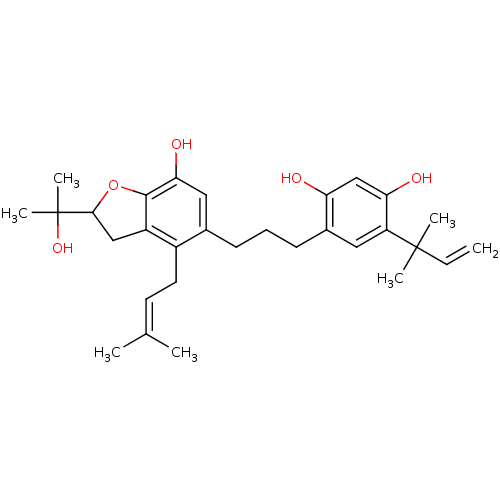 (5'-(2-methylbut-3-en-2-yl)-6''-(3-methylbut-2-enyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50241624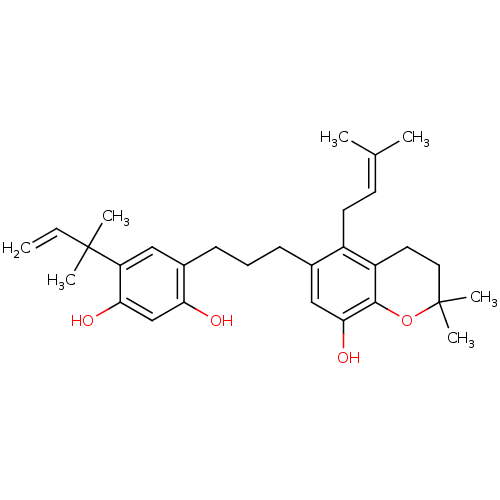 (CHEMBL454305 | Kazinol D) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50241624 (CHEMBL454305 | Kazinol D) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50253972 (5'-(2-methylbut-3-en-2-yl)-6''-(3-methylbut-2-enyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using as L-DOPA substrate | Bioorg Med Chem 17: 35-41 (2008) Article DOI: 10.1016/j.bmc.2008.11.022 BindingDB Entry DOI: 10.7270/Q2GH9HTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||