

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085045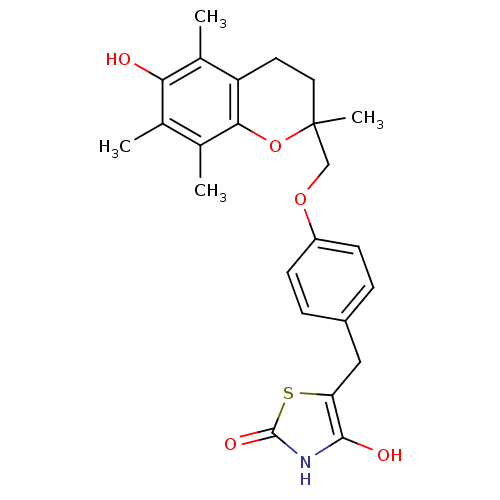 (5-((4-((6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant PPARgamma by Cheng-Prusoff equation based competitive binding TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.10.010 BindingDB Entry DOI: 10.7270/Q28919F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM24566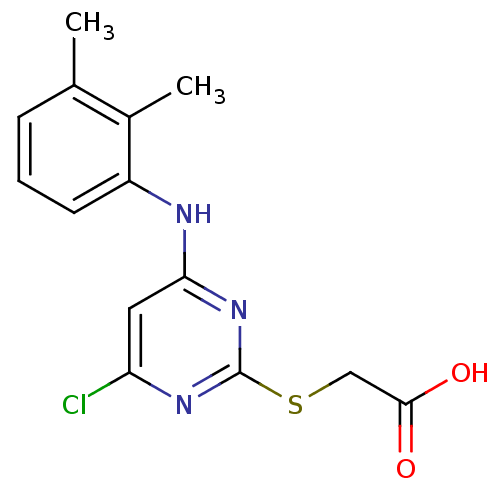 (2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant PPARalpha by Cheng-Prusoff equation based competitive binding TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.10.010 BindingDB Entry DOI: 10.7270/Q28919F4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50548252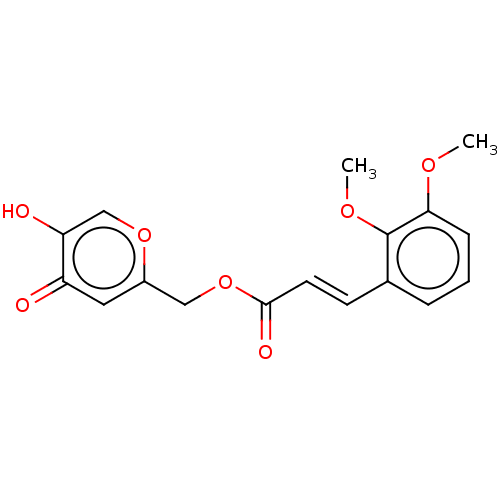 (CHEMBL4765137) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant PPARgamma by Cheng-Prusoff equation based competitive binding TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.10.010 BindingDB Entry DOI: 10.7270/Q28919F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50548251 (CHEMBL4797877) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant PPARgamma by Cheng-Prusoff equation based competitive binding TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.10.010 BindingDB Entry DOI: 10.7270/Q28919F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50548252 (CHEMBL4765137) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant PPARalpha by Cheng-Prusoff equation based competitive binding TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.10.010 BindingDB Entry DOI: 10.7270/Q28919F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50548251 (CHEMBL4797877) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant PPARalpha by Cheng-Prusoff equation based competitive binding TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.10.010 BindingDB Entry DOI: 10.7270/Q28919F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330115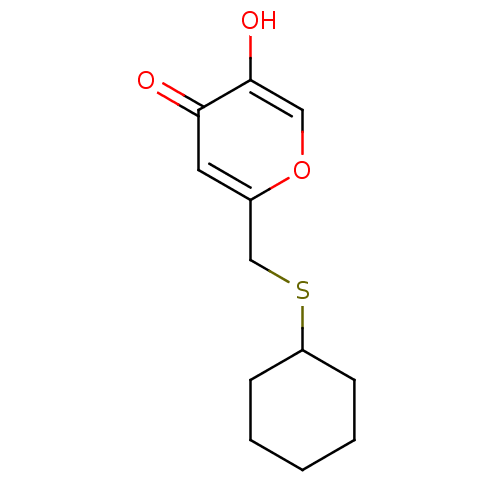 (2-(cyclohexylthiomethyl)-5-hydroxy-4H-pyran-4-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330111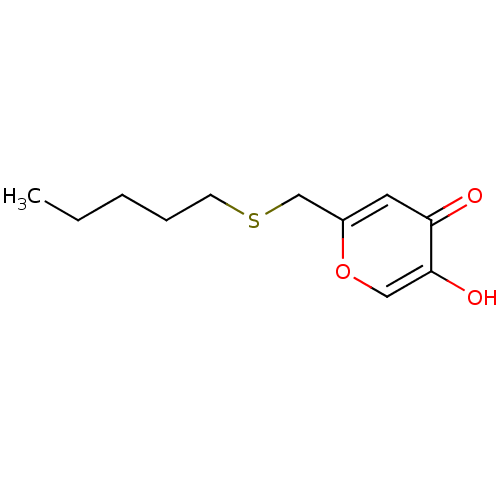 (5-hydroxy-2-(pentylthiomethyl)-4H-pyran-4-one | CH...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50248158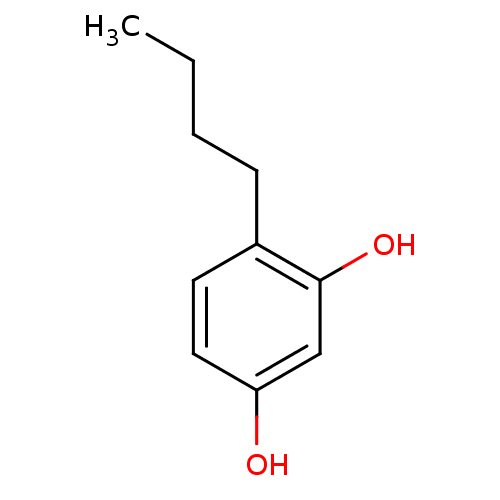 (CHEMBL450195 | N-butylresorcinol | US8993596, 4-Bu...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 20 mins | Bioorg Med Chem Lett 19: 1532-3 (2009) Article DOI: 10.1016/j.bmcl.2008.12.106 BindingDB Entry DOI: 10.7270/Q2KK9BNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330112 (2-(hexylthiomethyl)-5-hydroxy-4H-pyran-4-one | CHE...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50484888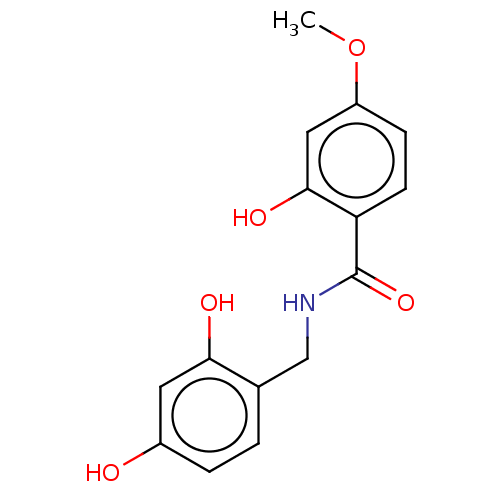 (CHEMBL2011797) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation R&D Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem Lett 22: 2110-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.144 BindingDB Entry DOI: 10.7270/Q2T72M9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50484884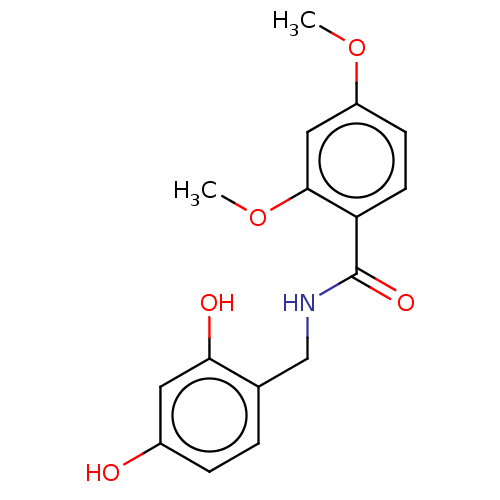 (CHEMBL2011798) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation R&D Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem Lett 22: 2110-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.144 BindingDB Entry DOI: 10.7270/Q2T72M9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50484882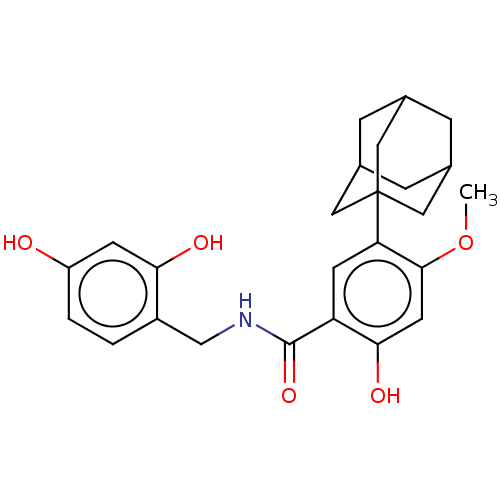 (CHEMBL2011795) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation R&D Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem Lett 22: 2110-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.144 BindingDB Entry DOI: 10.7270/Q2T72M9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50248201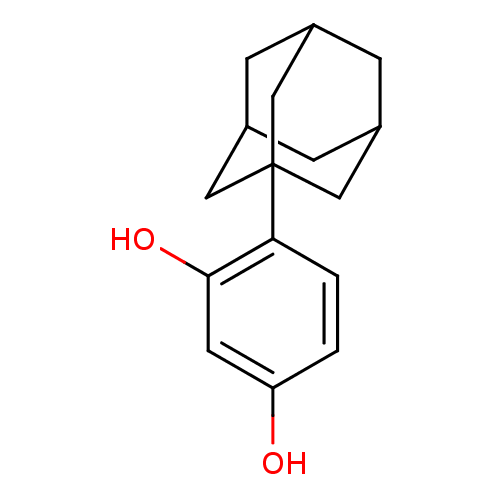 (4-adamantyl resorcinol | CHEMBL474953) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 20 mins | Bioorg Med Chem Lett 19: 1532-3 (2009) Article DOI: 10.1016/j.bmcl.2008.12.106 BindingDB Entry DOI: 10.7270/Q2KK9BNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50484886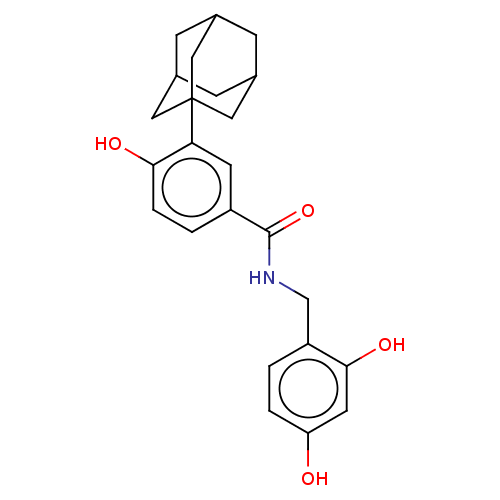 (CHEMBL2011799) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation R&D Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem Lett 22: 2110-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.144 BindingDB Entry DOI: 10.7270/Q2T72M9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50183707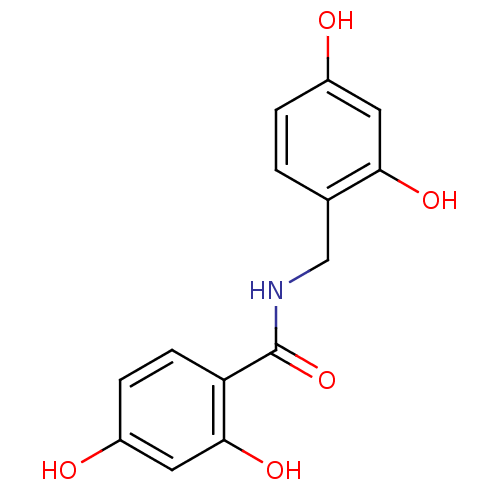 (CHEMBL205299 | N-(2,4-dihydroxybenzyl)-2,4-dihydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation R&D Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem Lett 22: 2110-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.144 BindingDB Entry DOI: 10.7270/Q2T72M9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50484883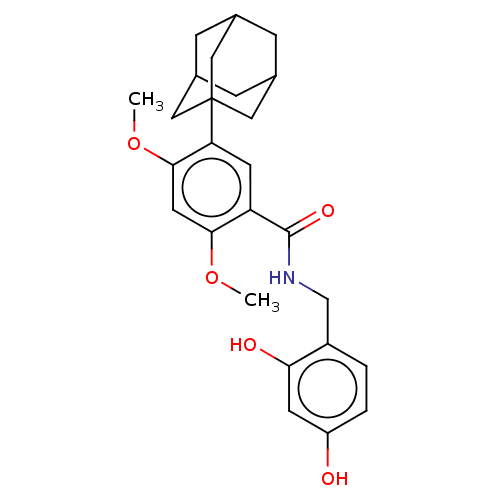 (CHEMBL2011796) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation R&D Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem Lett 22: 2110-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.144 BindingDB Entry DOI: 10.7270/Q2T72M9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50484887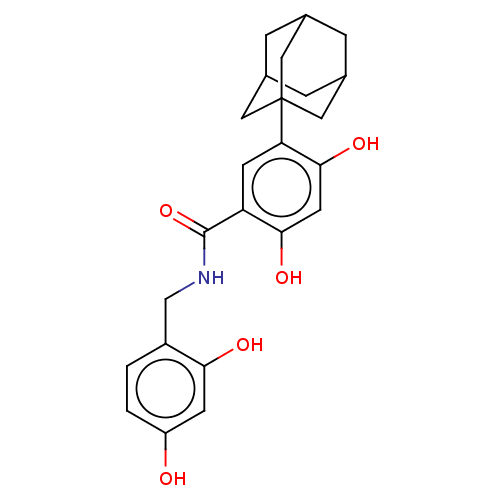 (CHEMBL2011794) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation R&D Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem Lett 22: 2110-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.144 BindingDB Entry DOI: 10.7270/Q2T72M9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50484885 (CHEMBL2011800) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation R&D Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem Lett 22: 2110-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.144 BindingDB Entry DOI: 10.7270/Q2T72M9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330110 (2-(butylthiomethyl)-5-hydroxy-4H-pyran-4-one | CHE...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50484889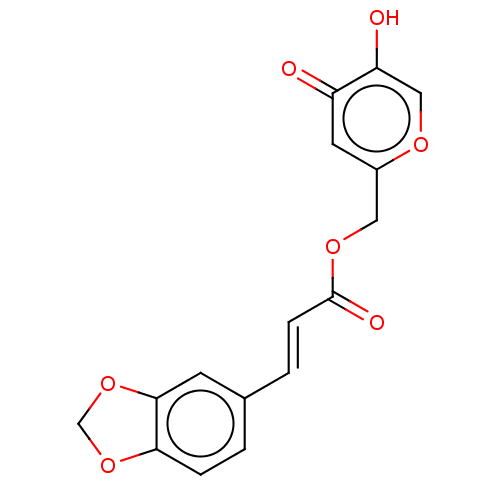 (CHEMBL2012553) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem Lett 22: 2004-7 (2012) Article DOI: 10.1016/j.bmcl.2012.01.032 BindingDB Entry DOI: 10.7270/Q2PK0K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50359632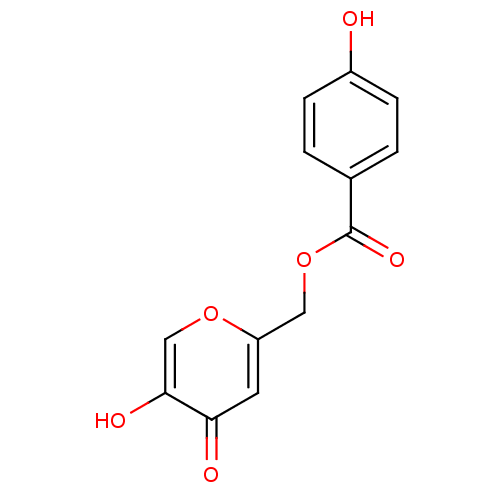 (CHEMBL1928641) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate after 20 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4159-62 (2012) Article DOI: 10.1016/j.bmcl.2012.04.046 BindingDB Entry DOI: 10.7270/Q23J3GV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330109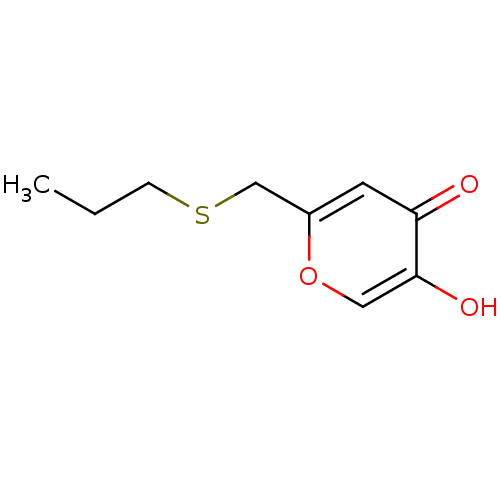 (5-hydroxy-2-(propylthiomethyl)-4H-pyran-4-one | CH...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50485146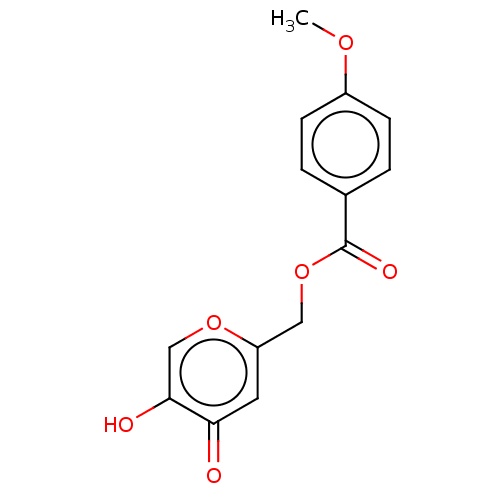 (CHEMBL2031845) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate after 20 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4159-62 (2012) Article DOI: 10.1016/j.bmcl.2012.04.046 BindingDB Entry DOI: 10.7270/Q23J3GV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50485146 (CHEMBL2031845) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate after 20 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4159-62 (2012) Article DOI: 10.1016/j.bmcl.2012.04.046 BindingDB Entry DOI: 10.7270/Q23J3GV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330108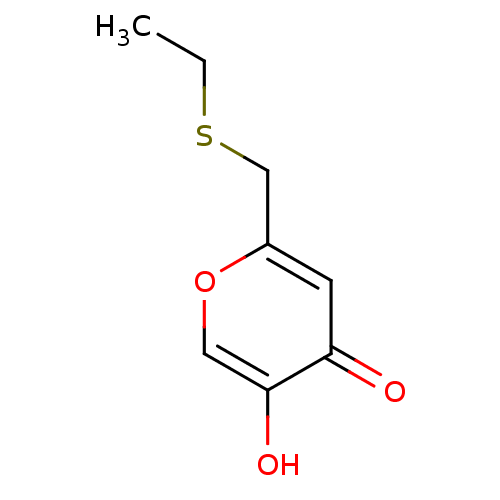 (2-(ethylthiomethyl)-5-hydroxy-4H-pyran-4-one | CHE...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330113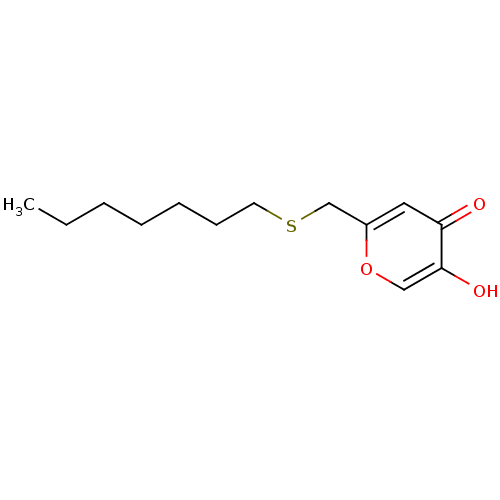 (2-(heptylthiomethyl)-5-hydroxy-4H-pyran-4-one | CH...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50359632 (CHEMBL1928641) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate after 20 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4159-62 (2012) Article DOI: 10.1016/j.bmcl.2012.04.046 BindingDB Entry DOI: 10.7270/Q23J3GV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50485139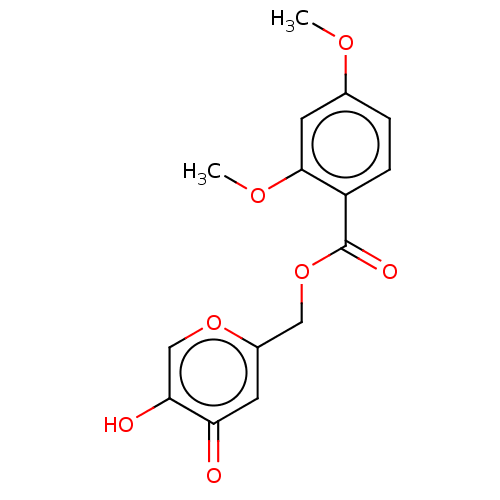 (CHEMBL2031848) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate after 20 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4159-62 (2012) Article DOI: 10.1016/j.bmcl.2012.04.046 BindingDB Entry DOI: 10.7270/Q23J3GV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50485145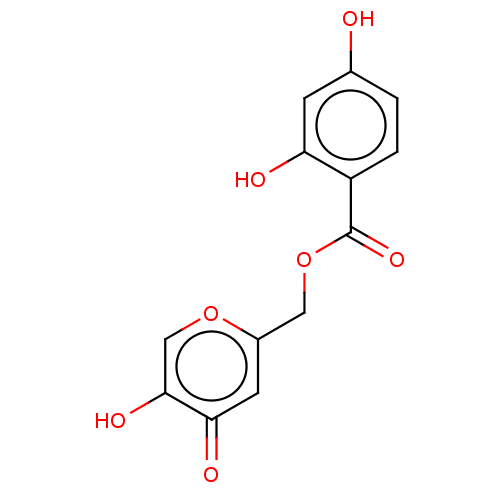 (CHEMBL2031846) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate after 20 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4159-62 (2012) Article DOI: 10.1016/j.bmcl.2012.04.046 BindingDB Entry DOI: 10.7270/Q23J3GV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50485145 (CHEMBL2031846) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate after 20 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4159-62 (2012) Article DOI: 10.1016/j.bmcl.2012.04.046 BindingDB Entry DOI: 10.7270/Q23J3GV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50485139 (CHEMBL2031848) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate after 20 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4159-62 (2012) Article DOI: 10.1016/j.bmcl.2012.04.046 BindingDB Entry DOI: 10.7270/Q23J3GV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50359630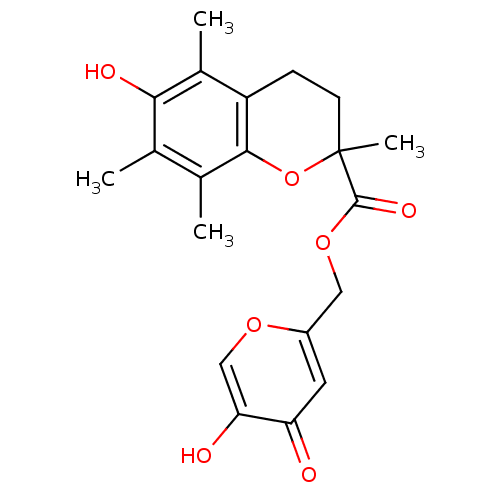 (CHEMBL1928639) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Skin Biotechnology Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosin as substrate after 20 mins | Bioorg Med Chem Lett 21: 7466-9 (2011) Article DOI: 10.1016/j.bmcl.2011.09.122 BindingDB Entry DOI: 10.7270/Q2DR2VZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50359632 (CHEMBL1928641) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Skin Biotechnology Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosin as substrate after 20 mins | Bioorg Med Chem Lett 21: 7466-9 (2011) Article DOI: 10.1016/j.bmcl.2011.09.122 BindingDB Entry DOI: 10.7270/Q2DR2VZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50359631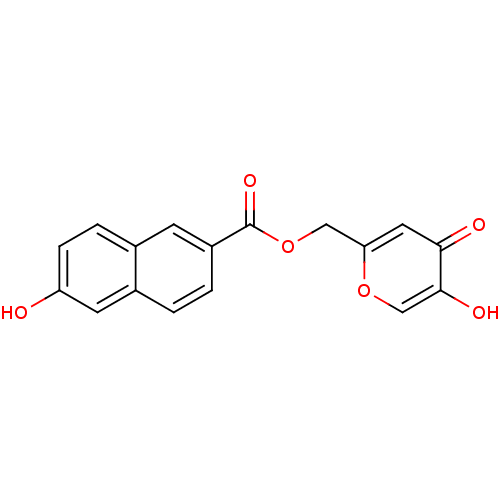 (CHEMBL1928640) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Skin Biotechnology Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosin as substrate after 20 mins | Bioorg Med Chem Lett 21: 7466-9 (2011) Article DOI: 10.1016/j.bmcl.2011.09.122 BindingDB Entry DOI: 10.7270/Q2DR2VZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM26190 (1,4-Dihydroxybenzene, XIII | 1,4-dihydroxybenzene ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 20 mins | Bioorg Med Chem Lett 19: 1532-3 (2009) Article DOI: 10.1016/j.bmcl.2008.12.106 BindingDB Entry DOI: 10.7270/Q2KK9BNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM26190 (1,4-Dihydroxybenzene, XIII | 1,4-dihydroxybenzene ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate after 20 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4159-62 (2012) Article DOI: 10.1016/j.bmcl.2012.04.046 BindingDB Entry DOI: 10.7270/Q23J3GV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50485140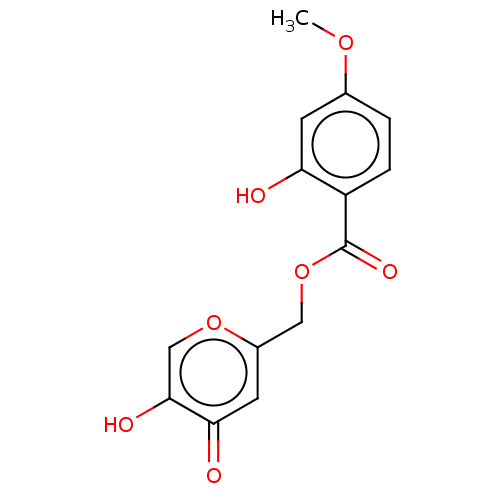 (CHEMBL2031847) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate after 20 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4159-62 (2012) Article DOI: 10.1016/j.bmcl.2012.04.046 BindingDB Entry DOI: 10.7270/Q23J3GV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50485140 (CHEMBL2031847) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate after 20 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4159-62 (2012) Article DOI: 10.1016/j.bmcl.2012.04.046 BindingDB Entry DOI: 10.7270/Q23J3GV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation R&D Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem Lett 22: 2110-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.144 BindingDB Entry DOI: 10.7270/Q2T72M9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330114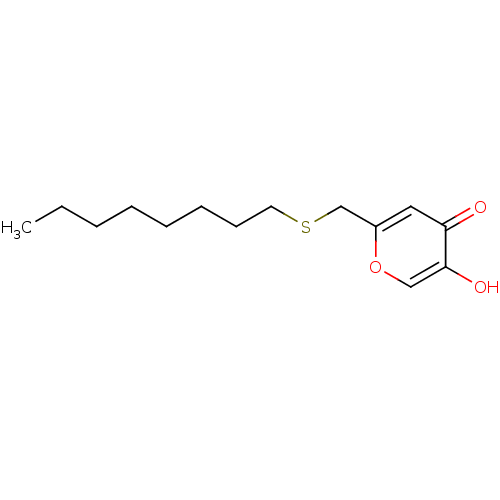 (5-hydroxy-2-(octylthiomethyl)-4H-pyran-4-one | CHE...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem Lett 22: 2004-7 (2012) Article DOI: 10.1016/j.bmcl.2012.01.032 BindingDB Entry DOI: 10.7270/Q2PK0K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330120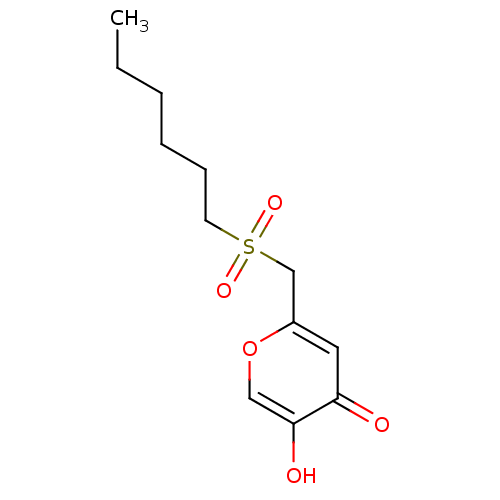 (2-(hexylsulfonylmethyl)-5-hydroxy-4H-pyran-4-one |...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate after 20 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4159-62 (2012) Article DOI: 10.1016/j.bmcl.2012.04.046 BindingDB Entry DOI: 10.7270/Q23J3GV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330121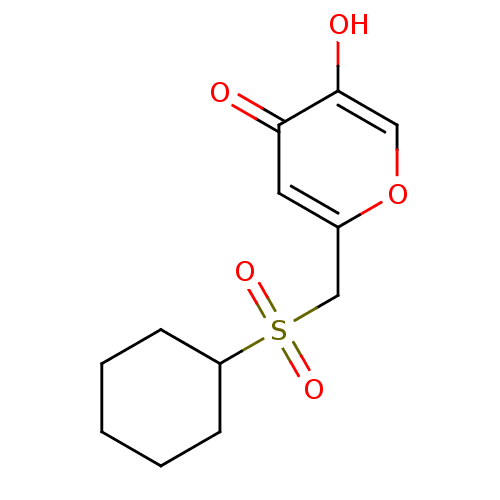 (2-(cyclohexylsulfonylmethyl)-5-hydroxy-4H-pyran-4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Skin Biotechnology Center Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity using L-tyrosin as substrate after 20 mins | Bioorg Med Chem Lett 21: 7466-9 (2011) Article DOI: 10.1016/j.bmcl.2011.09.122 BindingDB Entry DOI: 10.7270/Q2DR2VZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330117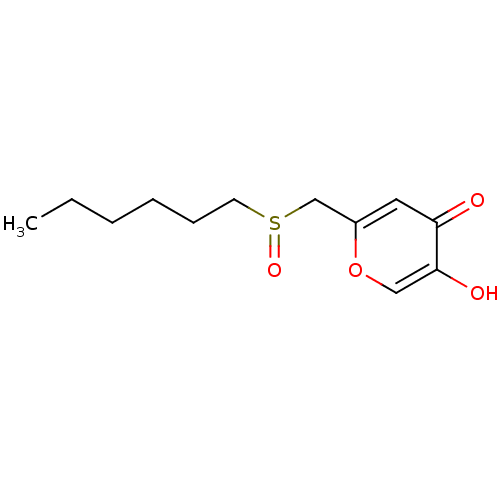 (2-(hexylsulfinylmethyl)-5-hydroxy-4H-pyran-4-one |...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330119 (5-hydroxy-2-(pentylsulfonylmethyl)-4H-pyran-4-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330118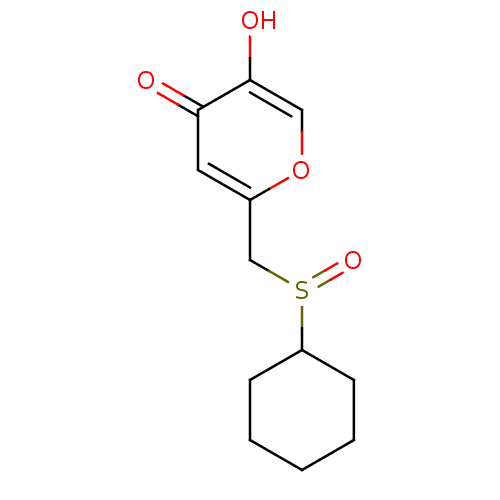 (2-(cyclohexylsulfinylmethyl)-5-hydroxy-4H-pyran-4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330116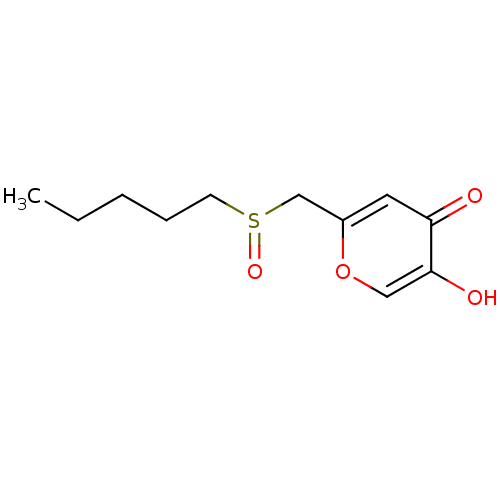 (5-hydroxy-2-(pentylsulfinylmethyl)-4H-pyran-4-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 20 mins by microplate reader analysis | Bioorg Med Chem Lett 20: 6569-71 (2010) Article DOI: 10.1016/j.bmcl.2010.09.042 BindingDB Entry DOI: 10.7270/Q2QF8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |