

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50044976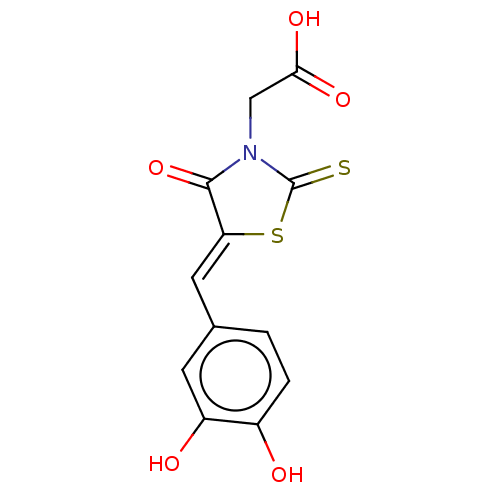 (CHEMBL2316879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Uncompetitive inhibition of His-tagged Escherichia coli DXR at 1 to 8 uM pre-incubated for 2 mins before reaction initiation in presence of 160 uM NA... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50153713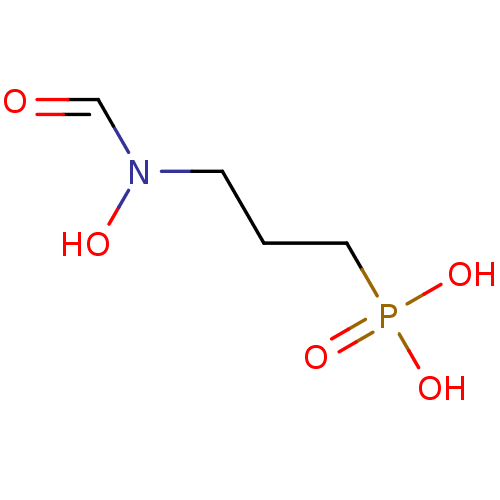 (3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of His-tagged Escherichia coli DXR pre-incubated for 2 mins before reaction initiation in presence of 160 uM NADPH in absence of 0.01% Tri... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50153713 (3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universite de Strasbourg | Assay Description Assays were performed in 50 mM Tris/HCl buffer, pH 7.5, containing 3 mM MgCl2 and 2 mM DTT. The concentrations of NADPH and DXP were 0.15 and 0.5 mM ... | Bioorg Chem 59: 140-4 (2015) Article DOI: 10.1016/j.bioorg.2015.02.008 BindingDB Entry DOI: 10.7270/Q2XS5T4J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium smegmatis (strain ATCC 700084 / mc(2...) | BDBM50153713 (3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Mycobacterium smegmatis DXR | Eur J Med Chem 51: 277-85 (2012) Article DOI: 10.1016/j.ejmech.2012.02.031 BindingDB Entry DOI: 10.7270/Q2474BWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium smegmatis (strain ATCC 700084 / mc(2...) | BDBM50181153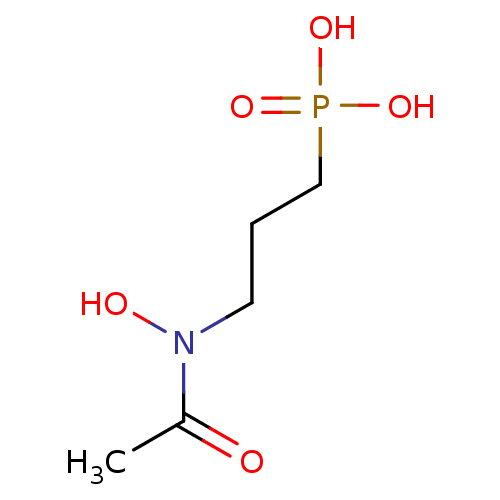 (3-(N-hydroxyacetamido)propylphosphonic acid | 3-(N...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Mycobacterium smegmatis DSM 43756 ATCC 19420 N-terminal his-tagged DXR expressed in XL1-blue Escherichia coli using NADPH and DXP as su... | Eur J Med Chem 51: 277-85 (2012) Article DOI: 10.1016/j.ejmech.2012.02.031 BindingDB Entry DOI: 10.7270/Q2474BWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Malate dehydrogenase (Thermus thermophilus) | BDBM59100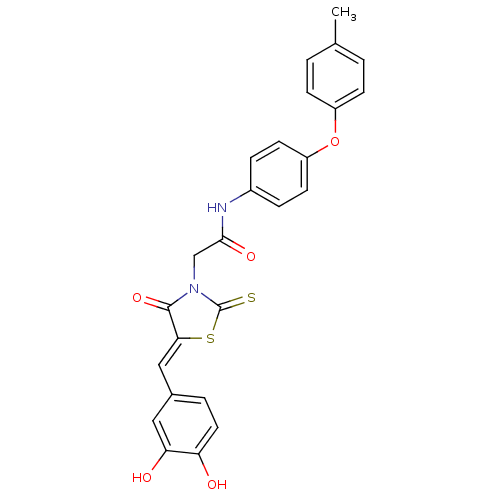 (Bi-ligand, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Thermus flavus MDH pre-incubated for 4 mins before reaction initiation in presence of OAA and NAPH in absence of 0.01% Triton X100 by U... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium smegmatis (strain ATCC 700084 / mc(2...) | BDBM50380000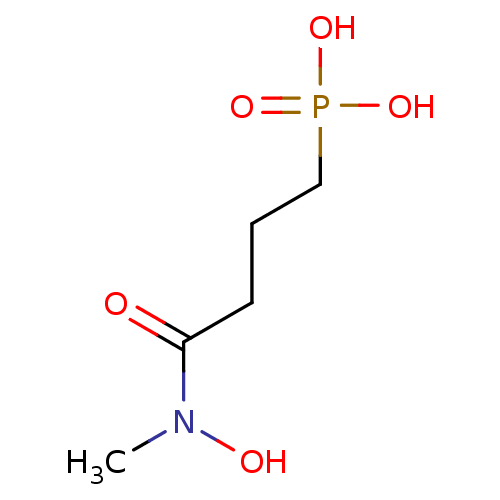 (CHEMBL258981) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Mycobacterium smegmatis DSM 43756 ATCC 19420 N-terminal his-tagged DXR expressed in XL1-blue Escherichia coli using NADPH and DXP as su... | Eur J Med Chem 51: 277-85 (2012) Article DOI: 10.1016/j.ejmech.2012.02.031 BindingDB Entry DOI: 10.7270/Q2474BWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Malate dehydrogenase (Thermus thermophilus) | BDBM50044967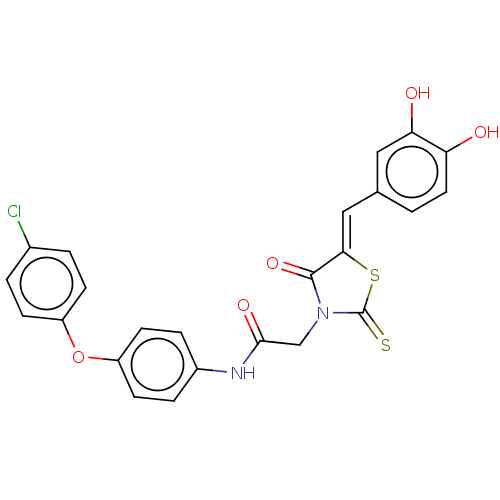 (CHEMBL3309759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Thermus flavus MDH pre-incubated for 4 mins before reaction initiation in presence of OAA and NAPH in absence of 0.01% Triton X100 by U... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium smegmatis (strain ATCC 700084 / mc(2...) | BDBM50153713 (3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Mycobacterium smegmatis DSM 43756 ATCC 19420 N-terminal his-tagged DXR expressed in XL1-blue Escherichia coli using NADPH and DXP as su... | Eur J Med Chem 51: 277-85 (2012) Article DOI: 10.1016/j.ejmech.2012.02.031 BindingDB Entry DOI: 10.7270/Q2474BWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Malate dehydrogenase (Thermus thermophilus) | BDBM50044973 (CHEMBL3309761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Thermus flavus MDH pre-incubated for 4 mins before reaction initiation in presence of OAA and NAPH in absence of 0.01% Triton X100 by U... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Malate dehydrogenase (Thermus thermophilus) | BDBM50044971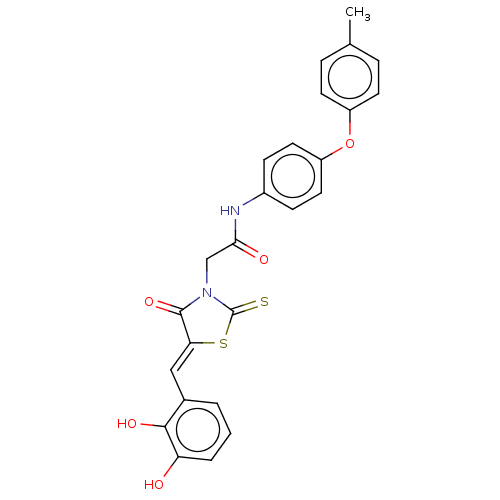 (CHEMBL3309760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Thermus flavus MDH pre-incubated for 4 mins before reaction initiation in presence of OAA and NAPH in absence of 0.01% Triton X100 by U... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Malate dehydrogenase (Thermus thermophilus) | BDBM50044974 (CHEMBL3309762) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Thermus flavus MDH pre-incubated for 4 mins before reaction initiation in presence of OAA and NAPH in absence of 0.01% Triton X100 by U... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium smegmatis (strain ATCC 700084 / mc(2...) | BDBM50379999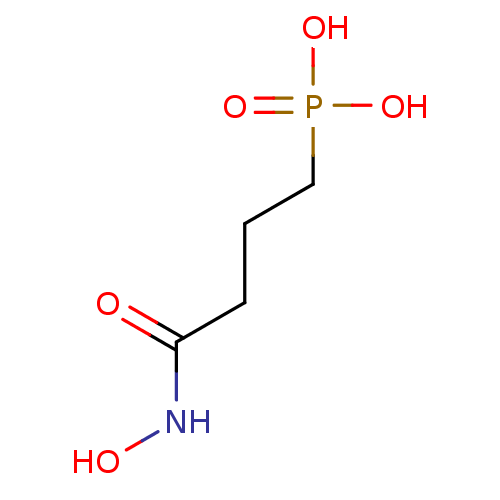 (CHEMBL1161784) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Mycobacterium smegmatis DSM 43756 ATCC 19420 N-terminal his-tagged DXR expressed in XL1-blue Escherichia coli using NADPH and DXP as su... | Eur J Med Chem 51: 277-85 (2012) Article DOI: 10.1016/j.ejmech.2012.02.031 BindingDB Entry DOI: 10.7270/Q2474BWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Strasbourg | Assay Description H-DXR was pre-incubated during 2 min in the presence of the inhibitors at different concentrations and DXP (480 然). NADPH (160 然 final concentratio... | Bioorg Chem 59: 140-4 (2015) Article DOI: 10.1016/j.bioorg.2015.02.008 BindingDB Entry DOI: 10.7270/Q2XS5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Strasbourg | Assay Description H-DXR was pre-incubated during 2 min in the presence of the inhibitors at different concentrations and DXP (480 然). NADPH (160 然 final concentratio... | Bioorg Chem 59: 140-4 (2015) Article DOI: 10.1016/j.bioorg.2015.02.008 BindingDB Entry DOI: 10.7270/Q2XS5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50009001 (5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Strasbourg | Assay Description H-DXR was pre-incubated during 2 min in the presence of the inhibitors at different concentrations and DXP (480 然). NADPH (160 然 final concentratio... | Bioorg Chem 59: 140-4 (2015) Article DOI: 10.1016/j.bioorg.2015.02.008 BindingDB Entry DOI: 10.7270/Q2XS5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50044976 (CHEMBL2316879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of His-tagged Escherichia coli DXR pre-incubated for 2 mins before reaction initiation in presence of 160 uM NADPH in absence of 0.01% Tri... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50044967 (CHEMBL3309759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of His-tagged Escherichia coli DXR pre-incubated for 2 mins before reaction initiation in presence of 160 uM NADPH in absence of 0.01% Tri... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50044973 (CHEMBL3309761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of His-tagged Escherichia coli DXR pre-incubated for 2 mins before reaction initiation in presence of 160 uM NADPH in absence of 0.01% Tri... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Strasbourg | Assay Description H-DXR was pre-incubated during 2 min in the presence of the inhibitors at different concentrations and DXP (480 然). NADPH (160 然 final concentratio... | Bioorg Chem 59: 140-4 (2015) Article DOI: 10.1016/j.bioorg.2015.02.008 BindingDB Entry DOI: 10.7270/Q2XS5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Malate dehydrogenase (Thermus thermophilus) | BDBM50044975 (CHEMBL3309763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Thermus flavus MDH pre-incubated for 4 mins before reaction initiation in presence of OAA and NAPH in absence of 0.01% Triton X100 by U... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM7457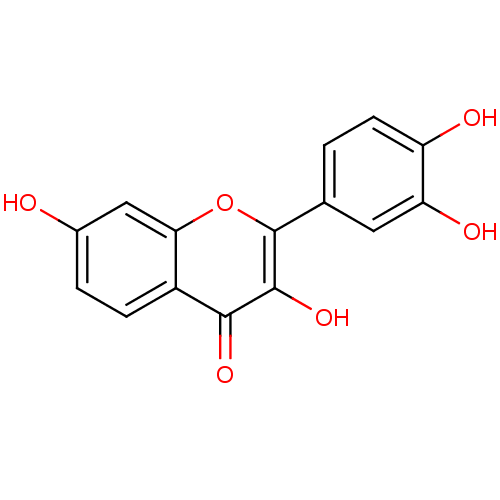 (2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-4H-chromen-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Strasbourg | Assay Description H-DXR was pre-incubated during 2 min in the presence of the inhibitors at different concentrations and DXP (480 然). NADPH (160 然 final concentratio... | Bioorg Chem 59: 140-4 (2015) Article DOI: 10.1016/j.bioorg.2015.02.008 BindingDB Entry DOI: 10.7270/Q2XS5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50044971 (CHEMBL3309760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of His-tagged Escherichia coli DXR pre-incubated for 2 mins before reaction initiation in presence of 160 uM NADPH in absence of 0.01% Tri... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50044975 (CHEMBL3309763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of His-tagged Escherichia coli DXR pre-incubated for 2 mins before reaction initiation in presence of 160 uM NADPH in absence of 0.01% Tri... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59100 (Bi-ligand, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of His-tagged Escherichia coli DXR pre-incubated for 2 mins before reaction initiation in presence of 160 uM NADPH in absence of 0.01% Tri... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50044976 (CHEMBL2316879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of His-tagged Escherichia coli DXR pre-incubated for 2 mins before reaction initiation in presence of 160 uM NADPH in presence of 0.01% Tr... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Malate dehydrogenase (Thermus thermophilus) | BDBM50044976 (CHEMBL2316879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Thermus flavus MDH pre-incubated for 4 mins before reaction initiation in presence of OAA and NAPH in absence of 0.01% Triton X100 by U... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50044975 (CHEMBL3309763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of His-tagged Escherichia coli DXR pre-incubated for 2 mins before reaction initiation in presence of 160 uM NADPH in presence of 0.01% Tr... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Malate dehydrogenase (Thermus thermophilus) | BDBM50044976 (CHEMBL2316879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of Thermus flavus MDH pre-incubated for 4 mins before reaction initiation in presence of OAA and NAPH in presence of 0.01% Triton X100 by ... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM50044974 (CHEMBL3309762) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS Curated by ChEMBL | Assay Description Inhibition of His-tagged Escherichia coli DXR pre-incubated for 2 mins before reaction initiation in presence of 160 uM NADPH in absence of 0.01% Tri... | Bioorg Med Chem 22: 3713-9 (2014) Article DOI: 10.1016/j.bmc.2014.05.004 BindingDB Entry DOI: 10.7270/Q2057HKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||