

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50246792 (CHEMBL508335 | N'-{3-(R)-[2-(S)-(1H-Indol-3-yl)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human recombinant pro-MMP2 by spectrofluorimeter | Bioorg Med Chem 16: 8745-59 (2008) Article DOI: 10.1016/j.bmc.2008.07.041 BindingDB Entry DOI: 10.7270/Q2R49QM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50609391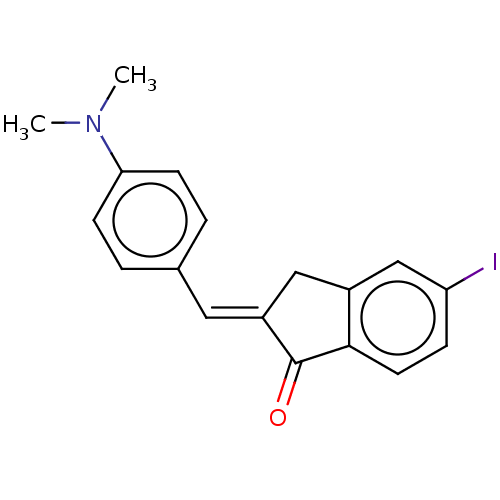 (CHEMBL5271649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50609382 (CHEMBL5274547) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50609382 (CHEMBL5274547) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50390102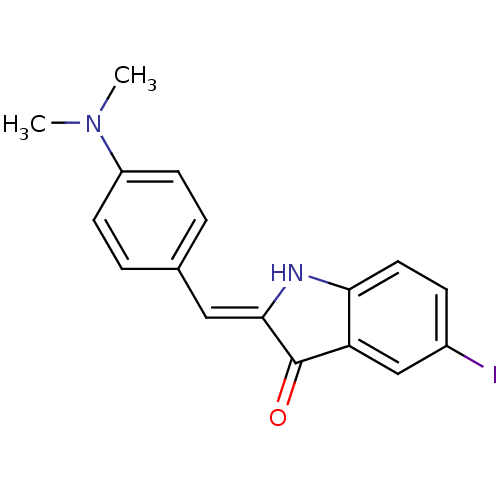 (CHEMBL2069433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50246792 (CHEMBL508335 | N'-{3-(R)-[2-(S)-(1H-Indol-3-yl)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 415 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP3 catalytic domain by spectrofluorimeter | Bioorg Med Chem 16: 8745-59 (2008) Article DOI: 10.1016/j.bmc.2008.07.041 BindingDB Entry DOI: 10.7270/Q2R49QM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50246792 (CHEMBL508335 | N'-{3-(R)-[2-(S)-(1H-Indol-3-yl)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 523 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human recombinant pro-MMP7 by spectrofluorimeter | Bioorg Med Chem 16: 8745-59 (2008) Article DOI: 10.1016/j.bmc.2008.07.041 BindingDB Entry DOI: 10.7270/Q2R49QM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50439491 (AUREUSIDIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50439491 (AUREUSIDIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50439491 (AUREUSIDIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50439491 (AUREUSIDIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50246792 (CHEMBL508335 | N'-{3-(R)-[2-(S)-(1H-Indol-3-yl)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP14 catalytic domain by spectrofluorimeter | Bioorg Med Chem 16: 8745-59 (2008) Article DOI: 10.1016/j.bmc.2008.07.041 BindingDB Entry DOI: 10.7270/Q2R49QM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human recombinant pro-MMP1 by spectrofluorimeter | Bioorg Med Chem 16: 8745-59 (2008) Article DOI: 10.1016/j.bmc.2008.07.041 BindingDB Entry DOI: 10.7270/Q2R49QM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human recombinant pro-MMP2 by spectrofluorimeter | Bioorg Med Chem 16: 8745-59 (2008) Article DOI: 10.1016/j.bmc.2008.07.041 BindingDB Entry DOI: 10.7270/Q2R49QM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of MMP9 | Bioorg Med Chem 15: 4753-66 (2007) Article DOI: 10.1016/j.bmc.2007.05.001 BindingDB Entry DOI: 10.7270/Q2Q23ZXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of human recombinant pro-MMP9 by spectrofluorimeter | Bioorg Med Chem 16: 8745-59 (2008) Article DOI: 10.1016/j.bmc.2008.07.041 BindingDB Entry DOI: 10.7270/Q2R49QM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM452998 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Incorporated US Patent | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | US Patent US10711007 (2020) BindingDB Entry DOI: 10.7270/Q2RR2286 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM452998 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM452999 ((2S,3S,4R,5R)-2-((R)-6- chloro-1,3- dihydroisobenz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Incorporated US Patent | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | US Patent US10711007 (2020) BindingDB Entry DOI: 10.7270/Q2RR2286 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM452998 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM452999 ((2S,3S,4R,5R)-2-((R)-6- chloro-1,3- dihydroisobenz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM452999 ((2S,3S,4R,5R)-2-((R)-6- chloro-1,3- dihydroisobenz...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of MMP2 | Bioorg Med Chem 15: 4753-66 (2007) Article DOI: 10.1016/j.bmc.2007.05.001 BindingDB Entry DOI: 10.7270/Q2Q23ZXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453006 ((2R,3R,4S,5S)-2-(4-amino-7H- pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453006 ((2R,3R,4S,5S)-2-(4-amino-7H- pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Incorporated US Patent | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | US Patent US10711007 (2020) BindingDB Entry DOI: 10.7270/Q2RR2286 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453006 ((2R,3R,4S,5S)-2-(4-amino-7H- pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453011 ((2R,3R,4S,5S)-2-(4-amino-7H- pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453011 ((2R,3R,4S,5S)-2-(4-amino-7H- pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453011 ((2R,3R,4S,5S)-2-(4-amino-7H- pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Incorporated US Patent | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | US Patent US10711007 (2020) BindingDB Entry DOI: 10.7270/Q2RR2286 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of MMP1 | Bioorg Med Chem 15: 4753-66 (2007) Article DOI: 10.1016/j.bmc.2007.05.001 BindingDB Entry DOI: 10.7270/Q2Q23ZXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM452997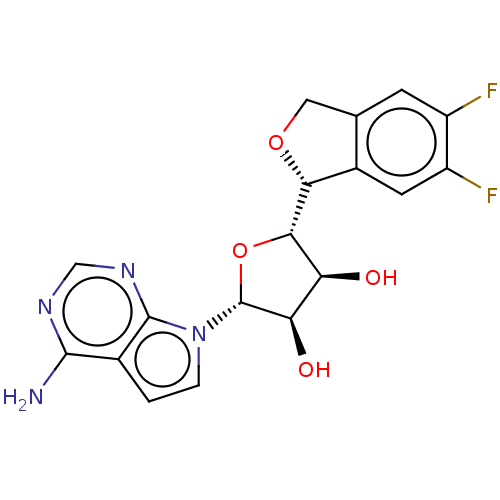 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM452997 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM452997 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Incorporated US Patent | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | US Patent US10711007 (2020) BindingDB Entry DOI: 10.7270/Q2RR2286 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453033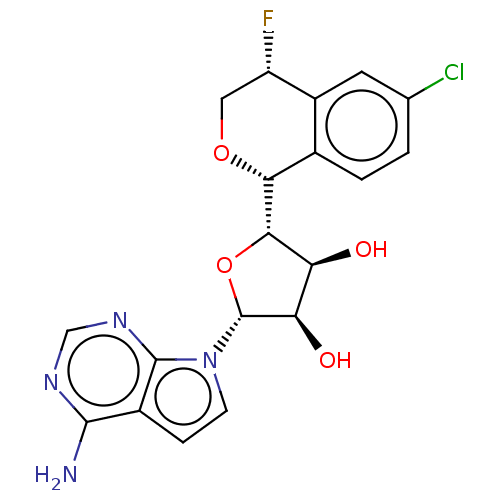 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Incorporated US Patent | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | US Patent US10711007 (2020) BindingDB Entry DOI: 10.7270/Q2RR2286 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453033 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453026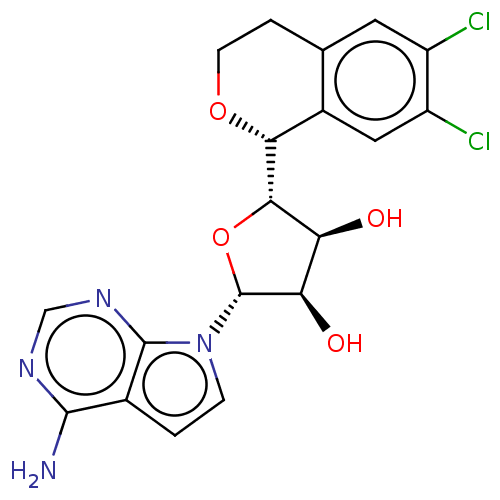 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453026 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Incorporated US Patent | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | US Patent US10711007 (2020) BindingDB Entry DOI: 10.7270/Q2RR2286 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50254779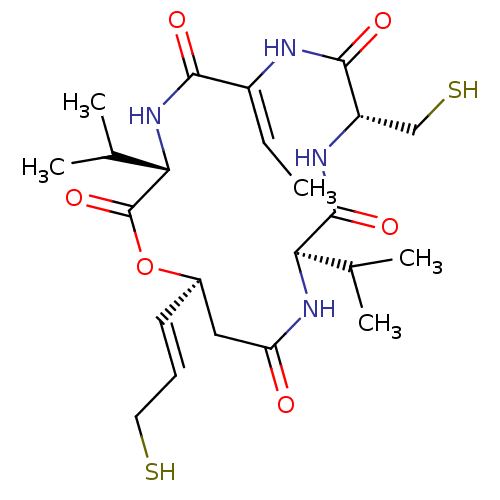 (CHEMBL4061025) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie Moléculaire de Reims, UMR 7312-CNRS, UFR Pharmacie , Université de Reims Champagne-Ardenne , 51 rue Cognacq-Jay , 51096 Reims Cedex , France. Curated by ChEMBL | Assay Description Inhibition of human HDAC1 expressed in human 293T cells preincubated for 15 mins in presence of [3H]acetyl-labeled histones measured after 30 mins by... | J Med Chem 61: 1745-1766 (2018) Article DOI: 10.1021/acs.jmedchem.7b00115 BindingDB Entry DOI: 10.7270/Q2FT8PGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453026 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453033 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Reims-Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of MMP3 | Bioorg Med Chem 15: 4753-66 (2007) Article DOI: 10.1016/j.bmc.2007.05.001 BindingDB Entry DOI: 10.7270/Q2Q23ZXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453024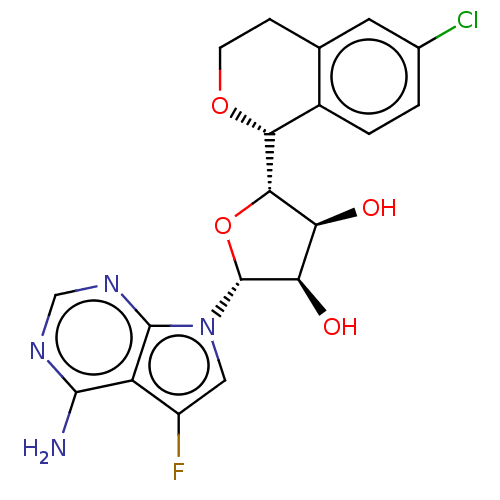 ((2R,3R,4S,5S)-2-(4-amino-5- fluoro-7H-pyrrolo[2,3-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453024 ((2R,3R,4S,5S)-2-(4-amino-5- fluoro-7H-pyrrolo[2,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453024 ((2R,3R,4S,5S)-2-(4-amino-5- fluoro-7H-pyrrolo[2,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Incorporated US Patent | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | US Patent US10711007 (2020) BindingDB Entry DOI: 10.7270/Q2RR2286 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453028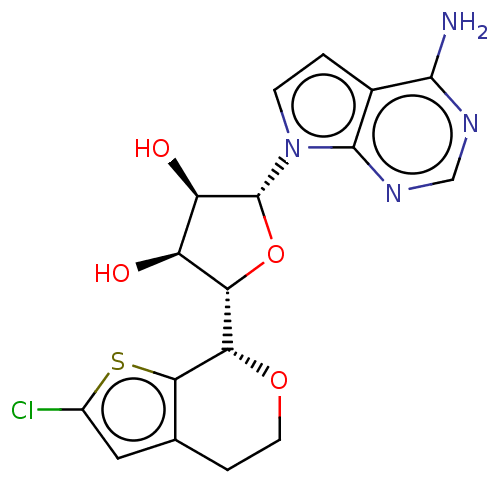 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Incorporated US Patent | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | US Patent US10711007 (2020) BindingDB Entry DOI: 10.7270/Q2RR2286 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453028 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453028 ((2R,3R,4S,5S)-2-(4-amino- 7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KS6VR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50609384 (CHEMBL5278614) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453015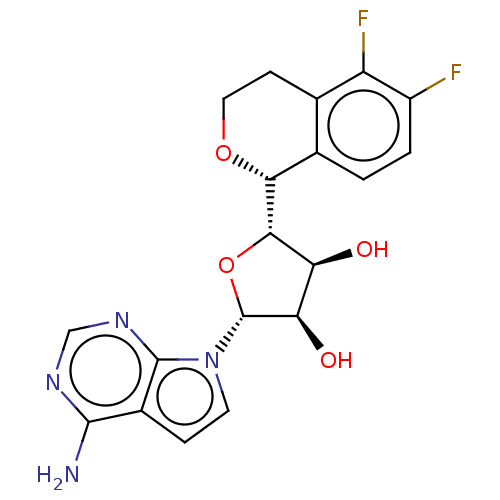 ((2R,3R,4S,5S)-2-(4-amino-7H- pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Incorporated US Patent | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | US Patent US10711007 (2020) BindingDB Entry DOI: 10.7270/Q2RR2286 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM453015 ((2R,3R,4S,5S)-2-(4-amino-7H- pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (20 mM Tris-HCl, pH 8.0,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 467 total ) | Next | Last >> |