 Found 54 hits with Last Name = 'rozamus' and Initial = 'lw'
Found 54 hits with Last Name = 'rozamus' and Initial = 'lw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36608

(Rapamycin C-7, analog 1)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42-,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36609
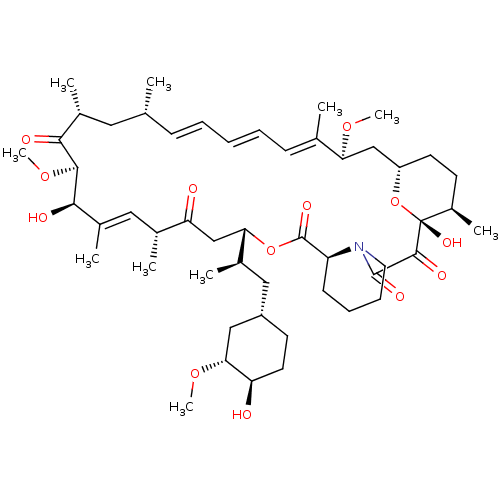
(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36627

(Rapamycin C-7, analog 16a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OCc2ccc(Cl)cc2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C57H82ClNO13/c1-34-15-11-10-12-16-35(2)48(70-33-41-19-22-43(58)23-20-41)31-44-24-18-40(7)57(67,72-44)54(64)55(65)59-26-14-13-17-45(59)56(66)71-49(37(4)29-42-21-25-46(60)50(30-42)68-8)32-47(61)36(3)28-39(6)52(63)53(69-9)51(62)38(5)27-34/h10-12,15-16,19-20,22-23,28,34,36-38,40,42,44-46,48-50,52-53,60,63,67H,13-14,17-18,21,24-27,29-33H2,1-9H3/b12-10+,15-11+,35-16+,39-28+/t34-,36-,37-,38-,40-,42+,44+,45+,46-,48-,49+,50-,52-,53+,57-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36623
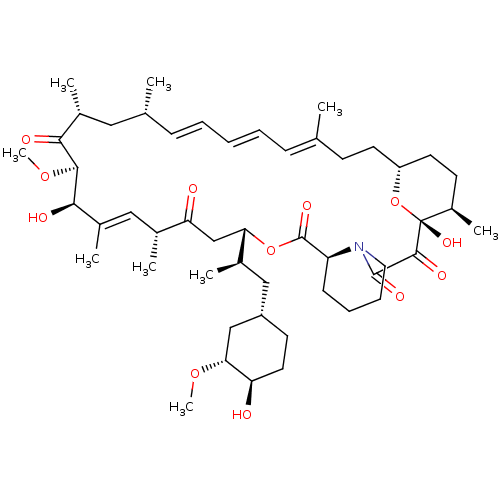
(Rapamycin C-7, analog 12)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\CC[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C50H77NO12/c1-30-15-11-10-12-16-31(2)25-34(5)44(54)46(61-9)45(55)35(6)26-32(3)41(53)29-42(33(4)27-37-20-23-40(52)43(28-37)60-8)62-49(58)39-17-13-14-24-51(39)48(57)47(56)50(59)36(7)19-22-38(63-50)21-18-30/h10-12,15-16,26,31-34,36-40,42-43,45-46,52,55,59H,13-14,17-25,27-29H2,1-9H3/b11-10+,16-12+,30-15+,35-26+/t31-,32-,33-,34-,36-,37+,38-,39+,40-,42+,43-,45-,46+,50-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36612
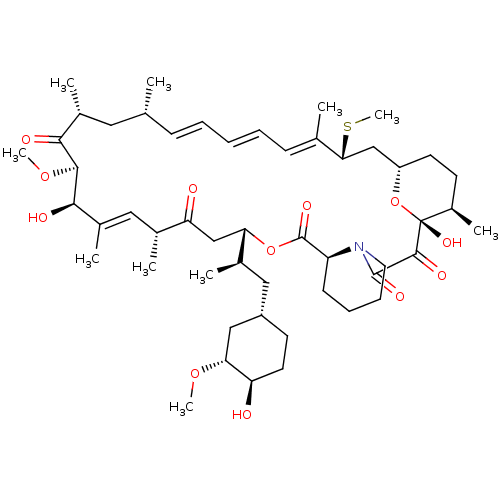
(Rapamycin C-7, analog 6a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)SC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO12S/c1-30-16-12-11-13-17-31(2)44(65-10)28-38-21-19-36(7)51(60,64-38)48(57)49(58)52-23-15-14-18-39(52)50(59)63-42(33(4)26-37-20-22-40(53)43(27-37)61-8)29-41(54)32(3)25-35(6)46(56)47(62-9)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43-,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36613
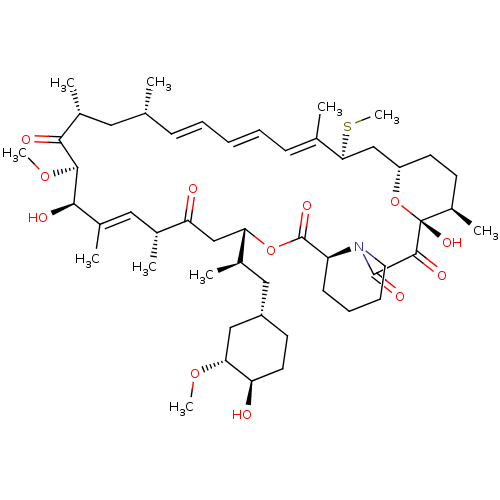
(Rapamycin C-7, analog 6b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)SC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO12S/c1-30-16-12-11-13-17-31(2)44(65-10)28-38-21-19-36(7)51(60,64-38)48(57)49(58)52-23-15-14-18-39(52)50(59)63-42(33(4)26-37-20-22-40(53)43(27-37)61-8)29-41(54)32(3)25-35(6)46(56)47(62-9)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43-,44+,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36622

(Rapamycin C-7, analog 11b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccc[nH]2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C54H80N2O12/c1-32-16-11-10-12-17-33(2)41(42-18-15-24-55-42)30-40-22-20-38(7)54(64,68-40)51(61)52(62)56-25-14-13-19-43(56)53(63)67-46(35(4)28-39-21-23-44(57)47(29-39)65-8)31-45(58)34(3)27-37(6)49(60)50(66-9)48(59)36(5)26-32/h10-12,15-18,24,27,32,34-36,38-41,43-44,46-47,49-50,55,57,60,64H,13-14,19-23,25-26,28-31H2,1-9H3/b12-10+,16-11+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43+,44-,46+,47-,49-,50+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36619
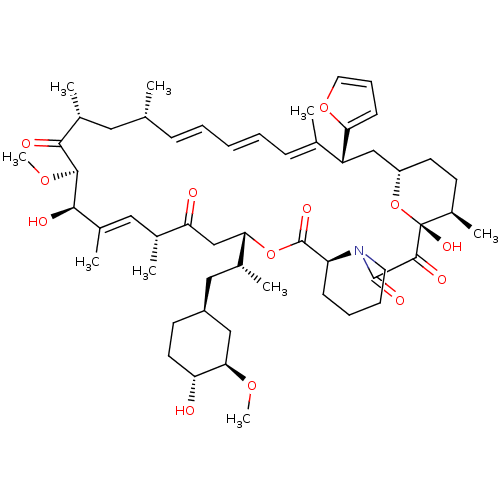
(Rapamycin C-7, analog 10a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccco2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C54H79NO13/c1-32-16-11-10-12-17-33(2)41(45-19-15-25-66-45)30-40-22-20-38(7)54(63,68-40)51(60)52(61)55-24-14-13-18-42(55)53(62)67-46(35(4)28-39-21-23-43(56)47(29-39)64-8)31-44(57)34(3)27-37(6)49(59)50(65-9)48(58)36(5)26-32/h10-12,15-17,19,25,27,32,34-36,38-43,46-47,49-50,56,59,63H,13-14,18,20-24,26,28-31H2,1-9H3/b12-10+,16-11+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41-,42+,43-,46+,47-,49-,50+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36610
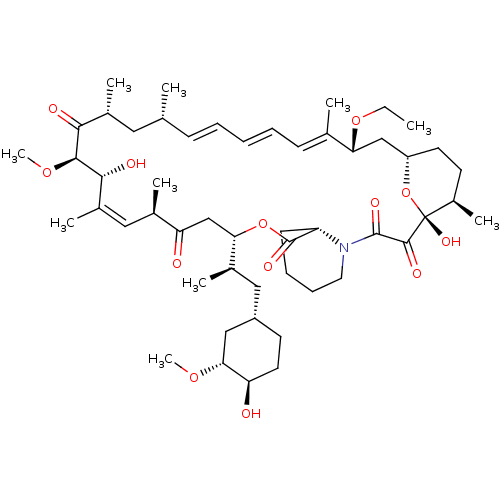
(Rapamycin C-7, analog 5a)Show SMILES CCO[C@@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H](CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:34,t:49,51,53| Show InChI InChI=1S/C52H81NO13/c1-11-64-43-29-39-22-20-37(8)52(61,66-39)49(58)50(59)53-24-16-15-19-40(53)51(60)65-44(34(5)27-38-21-23-41(54)45(28-38)62-9)30-42(55)33(4)26-36(7)47(57)48(63-10)46(56)35(6)25-31(2)17-13-12-14-18-32(43)3/h12-14,17-18,26,31,33-35,37-41,43-45,47-48,54,57,61H,11,15-16,19-25,27-30H2,1-10H3/b14-12+,17-13+,32-18+,36-26+/t31-,33-,34-,35-,37-,38+,39+,40+,41-,43-,44+,45-,47-,48+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36615
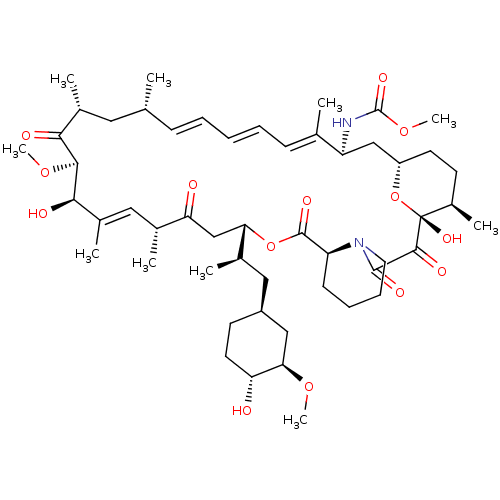
(Rapamycin C-7, analog 7b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)NC(=O)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C52H80N2O14/c1-30-16-12-11-13-17-31(2)39(53-51(62)66-10)28-38-21-19-36(7)52(63,68-38)48(59)49(60)54-23-15-14-18-40(54)50(61)67-43(33(4)26-37-20-22-41(55)44(27-37)64-8)29-42(56)32(3)25-35(6)46(58)47(65-9)45(57)34(5)24-30/h11-13,16-17,25,30,32-34,36-41,43-44,46-47,55,58,63H,14-15,18-24,26-29H2,1-10H3,(H,53,62)/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40+,41-,43+,44-,46-,47+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36611

(Rapamycin C-7, analog 5b)Show SMILES CCO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H](CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:34,t:49,51,53| Show InChI InChI=1S/C52H81NO13/c1-11-64-43-29-39-22-20-37(8)52(61,66-39)49(58)50(59)53-24-16-15-19-40(53)51(60)65-44(34(5)27-38-21-23-41(54)45(28-38)62-9)30-42(55)33(4)26-36(7)47(57)48(63-10)46(56)35(6)25-31(2)17-13-12-14-18-32(43)3/h12-14,17-18,26,31,33-35,37-41,43-45,47-48,54,57,61H,11,15-16,19-25,27-30H2,1-10H3/b14-12+,17-13+,32-18+,36-26+/t31-,33-,34-,35-,37-,38+,39+,40+,41-,43+,44+,45-,47-,48+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36614
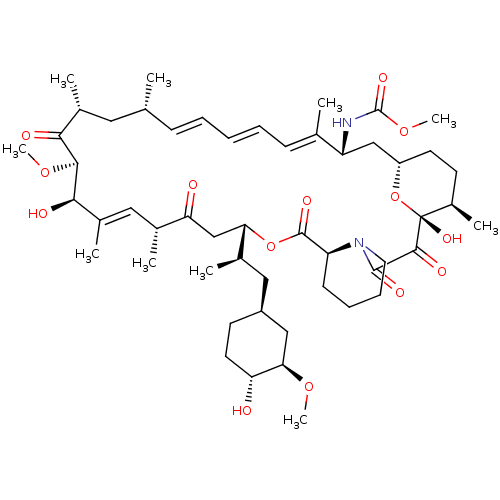
(Rapamycin C-7, analog 7a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)NC(=O)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C52H80N2O14/c1-30-16-12-11-13-17-31(2)39(53-51(62)66-10)28-38-21-19-36(7)52(63,68-38)48(59)49(60)54-23-15-14-18-40(54)50(61)67-43(33(4)26-37-20-22-41(55)44(27-37)64-8)29-42(56)32(3)25-35(6)46(58)47(65-9)45(57)34(5)24-30/h11-13,16-17,25,30,32-34,36-41,43-44,46-47,55,58,63H,14-15,18-24,26-29H2,1-10H3,(H,53,62)/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39-,40+,41-,43+,44-,46-,47+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36625
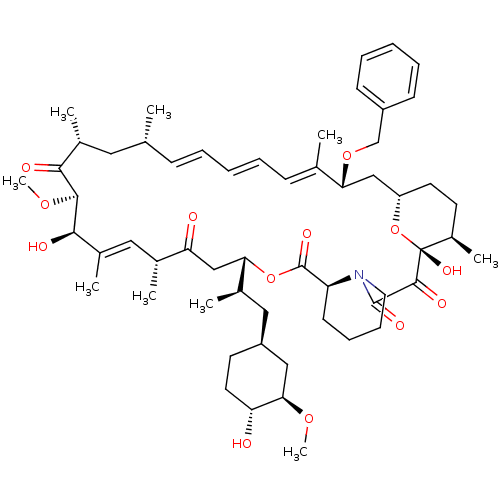
(Rapamycin C-7, analog 14a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OCc2ccccc2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C57H83NO13/c1-35-18-12-10-13-19-36(2)48(69-34-42-20-14-11-15-21-42)32-44-25-23-41(7)57(66,71-44)54(63)55(64)58-27-17-16-22-45(58)56(65)70-49(38(4)30-43-24-26-46(59)50(31-43)67-8)33-47(60)37(3)29-40(6)52(62)53(68-9)51(61)39(5)28-35/h10-15,18-21,29,35,37-39,41,43-46,48-50,52-53,59,62,66H,16-17,22-28,30-34H2,1-9H3/b13-10+,18-12+,36-19+,40-29+/t35-,37-,38-,39-,41-,43+,44+,45+,46-,48-,49+,50-,52-,53+,57-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36620

(Rapamycin C-7, analog 10b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccco2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C54H79NO13/c1-32-16-11-10-12-17-33(2)41(45-19-15-25-66-45)30-40-22-20-38(7)54(63,68-40)51(60)52(61)55-24-14-13-18-42(55)53(62)67-46(35(4)28-39-21-23-43(56)47(29-39)64-8)31-44(57)34(3)27-37(6)49(59)50(65-9)48(58)36(5)26-32/h10-12,15-17,19,25,27,32,34-36,38-43,46-47,49-50,56,59,63H,13-14,18,20-24,26,28-31H2,1-9H3/b12-10+,16-11+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,42+,43-,46+,47-,49-,50+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36618

(Rapamycin C-7, analog 9)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2c(OC)cc(OC)cc2OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C59H87NO15/c1-34-18-14-13-15-19-35(2)44(52-50(71-10)31-43(69-8)32-51(52)72-11)30-42-23-21-40(7)59(68,75-42)56(65)57(66)60-25-17-16-20-45(60)58(67)74-48(37(4)28-41-22-24-46(61)49(29-41)70-9)33-47(62)36(3)27-39(6)54(64)55(73-12)53(63)38(5)26-34/h13-15,18-19,27,31-32,34,36-38,40-42,44-46,48-49,54-55,61,64,68H,16-17,20-26,28-30,33H2,1-12H3/b15-13+,18-14+,35-19+,39-27+/t34-,36-,37-,38-,40-,41+,42+,44+,45+,46-,48+,49-,54-,55+,59-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36626

(Rapamycin C-7, analog 15a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OCc2cccc(c2)N(=O)=O)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C57H82N2O15/c1-34-16-11-10-12-17-35(2)48(72-33-42-18-15-19-43(29-42)59(68)69)31-44-23-21-40(7)57(67,74-44)54(64)55(65)58-25-14-13-20-45(58)56(66)73-49(37(4)28-41-22-24-46(60)50(30-41)70-8)32-47(61)36(3)27-39(6)52(63)53(71-9)51(62)38(5)26-34/h10-12,15-19,27,29,34,36-38,40-41,44-46,48-50,52-53,60,63,67H,13-14,20-26,28,30-33H2,1-9H3/b12-10+,16-11+,35-17+,39-27+/t34-,36-,37-,38-,40-,41+,44+,45+,46-,48-,49+,50-,52-,53+,57-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36616

(Rapamycin C-7, analog 8a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccc(OC)cc2OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C58H85NO14/c1-34-17-13-12-14-18-35(2)45(44-24-23-42(68-8)32-50(44)69-9)31-43-22-20-40(7)58(67,73-43)55(64)56(65)59-26-16-15-19-46(59)57(66)72-49(37(4)29-41-21-25-47(60)51(30-41)70-10)33-48(61)36(3)28-39(6)53(63)54(71-11)52(62)38(5)27-34/h12-14,17-18,23-24,28,32,34,36-38,40-41,43,45-47,49,51,53-54,60,63,67H,15-16,19-22,25-27,29-31,33H2,1-11H3/b14-12+,17-13+,35-18+,39-28+/t34-,36-,37-,38-,40-,41+,43+,45-,46+,47-,49+,51-,53-,54+,58-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36621

(Rapamycin C-7, analog 11a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccc[nH]2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C54H80N2O12/c1-32-16-11-10-12-17-33(2)41(42-18-15-24-55-42)30-40-22-20-38(7)54(64,68-40)51(61)52(62)56-25-14-13-19-43(56)53(63)67-46(35(4)28-39-21-23-44(57)47(29-39)65-8)31-45(58)34(3)27-37(6)49(60)50(66-9)48(59)36(5)26-32/h10-12,15-18,24,27,32,34-36,38-41,43-44,46-47,49-50,55,57,60,64H,13-14,19-23,25-26,28-31H2,1-9H3/b12-10+,16-11+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41-,43+,44-,46+,47-,49-,50+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36624
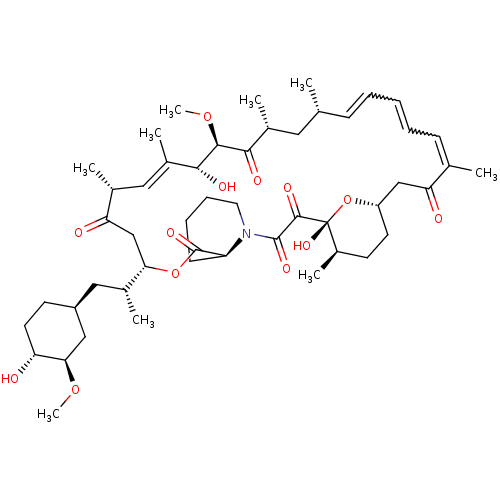
(Rapamycin C-7, analog 13)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=CC=C(C)C(=O)C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)CC[C@H]1O |w:31.30,33.32,c:14,t:29| Show InChI InChI=1S/C50H75NO13/c1-29-15-11-10-12-16-30(2)40(53)27-37-20-18-35(7)50(60,64-37)47(57)48(58)51-22-14-13-17-38(51)49(59)63-42(32(4)25-36-19-21-39(52)43(26-36)61-8)28-41(54)31(3)24-34(6)45(56)46(62-9)44(55)33(5)23-29/h10-12,15-16,24,29,31-33,35-39,42-43,45-46,52,56,60H,13-14,17-23,25-28H2,1-9H3/b12-10?,15-11+,30-16?,34-24+/t29-,31-,32-,33-,35-,36+,37+,38+,39-,42+,43-,45-,46+,50-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36628

(Rapamycin C-7, analog 17a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OCc2ccc(N=[N]#N)c(I)c2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C57H81IN4O13/c1-33-15-11-10-12-16-34(2)48(73-32-41-19-22-44(60-61-59)43(58)28-41)30-42-21-18-39(7)57(70,75-42)54(67)55(68)62-24-14-13-17-45(62)56(69)74-49(36(4)27-40-20-23-46(63)50(29-40)71-8)31-47(64)35(3)26-38(6)52(66)53(72-9)51(65)37(5)25-33/h10-12,15-16,19,22,26,28,33,35-37,39-40,42,45-46,48-50,52-53,63,66,70H,13-14,17-18,20-21,23-25,27,29-32H2,1-9H3/b12-10+,15-11+,34-16+,38-26+/t33-,35-,36-,37-,39-,40+,42+,45+,46-,48-,49+,50-,52-,53+,57-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36617
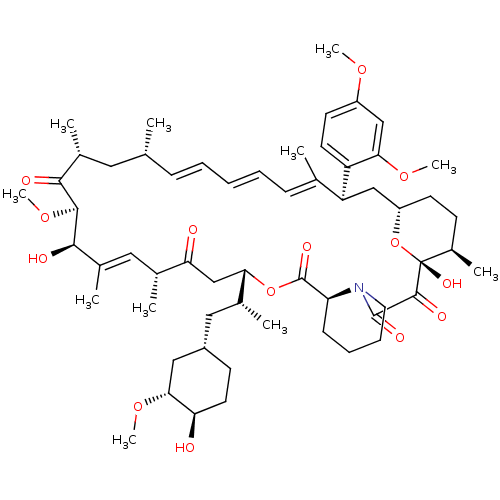
(Rapamycin C-7, analog 8b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccc(OC)cc2OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C58H85NO14/c1-34-17-13-12-14-18-35(2)45(44-24-23-42(68-8)32-50(44)69-9)31-43-22-20-40(7)58(67,73-43)55(64)56(65)59-26-16-15-19-46(59)57(66)72-49(37(4)29-41-21-25-47(60)51(30-41)70-10)33-48(61)36(3)28-39(6)53(63)54(71-11)52(62)38(5)27-34/h12-14,17-18,23-24,28,32,34,36-38,40-41,43,45-47,49,51,53-54,60,63,67H,15-16,19-22,25-27,29-31,33H2,1-11H3/b14-12+,17-13+,35-18+,39-28+/t34-,36-,37-,38-,40-,41+,43+,45+,46+,47-,49+,51-,53-,54+,58-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086083

(1-[2-(3,4,5-Trimethoxy-phenyl)-pent-4-enoyl]-piper...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](CC=C)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H47NO11/c1-7-11-29(27-22-34(47-4)37(49-6)35(23-27)48-5)38(43)40-19-9-8-14-30(40)39(44)51-31(26-12-10-13-28(21-26)50-24-36(41)42)17-15-25-16-18-32(45-2)33(20-25)46-3/h7,10,12-13,16,18,20-23,29-31H,1,8-9,11,14-15,17,19,24H2,2-6H3,(H,41,42)/t29-,30-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086090

(1-[2-(3,4,5-Trimethoxy-phenyl)-butyryl]-piperidine...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)OC(CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C38H47NO11/c1-7-28(26-21-33(46-4)36(48-6)34(22-26)47-5)37(42)39-18-9-8-13-29(39)38(43)50-30(25-11-10-12-27(20-25)49-23-35(40)41)16-14-24-15-17-31(44-2)32(19-24)45-3/h10-12,15,17,19-22,28-30H,7-9,13-14,16,18,23H2,1-6H3,(H,40,41)/t28-,29-,30?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086094

(1-[2-(3,4,5-Trimethoxy-phenyl)-pentanoyl]-piperidi...)Show SMILES CCC[C@H](C(=O)N1CCCC[C@H]1C(=O)OC(CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C39H49NO11/c1-7-11-29(27-22-34(47-4)37(49-6)35(23-27)48-5)38(43)40-19-9-8-14-30(40)39(44)51-31(26-12-10-13-28(21-26)50-24-36(41)42)17-15-25-16-18-32(45-2)33(20-25)46-3/h10,12-13,16,18,20-23,29-31H,7-9,11,14-15,17,19,24H2,1-6H3,(H,41,42)/t29-,30-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086077
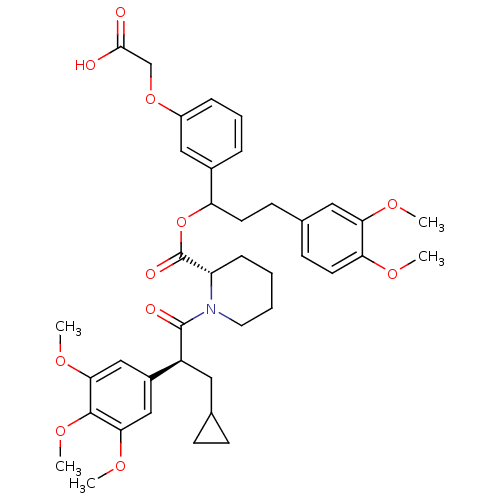
(1-[3-Cyclopropyl-2-(3,4,5-trimethoxy-phenyl)-propi...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](CC2CC2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C40H49NO11/c1-46-33-17-15-26(20-34(33)47-2)14-16-32(27-9-8-10-29(21-27)51-24-37(42)43)52-40(45)31-11-6-7-18-41(31)39(44)30(19-25-12-13-25)28-22-35(48-3)38(50-5)36(23-28)49-4/h8-10,15,17,20-23,25,30-32H,6-7,11-14,16,18-19,24H2,1-5H3,(H,42,43)/t30-,31-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132554

((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-butyryl]-pi...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C36H43NO9/c1-5-28(26-21-31(42-2)34(44-4)32(22-26)43-3)35(40)37-19-10-9-16-29(37)36(41)46-30(18-17-24-12-7-6-8-13-24)25-14-11-15-27(20-25)45-23-33(38)39/h6-8,11-15,20-22,28-30H,5,9-10,16-19,23H2,1-4H3,(H,38,39)/t28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086092

(1-[3-Methyl-2-(3,4,5-trimethoxy-phenyl)-butyryl]-p...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](C(C)C)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H49NO11/c1-24(2)36(27-21-33(47-5)37(49-7)34(22-27)48-6)38(43)40-18-9-8-13-29(40)39(44)51-30(26-11-10-12-28(20-26)50-23-35(41)42)16-14-25-15-17-31(45-3)32(19-25)46-4/h10-12,15,17,19-22,24,29-30,36H,8-9,13-14,16,18,23H2,1-7H3,(H,41,42)/t29-,30?,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132541

((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-butyryl]-pi...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C38H47NO11/c1-7-28(26-21-33(46-4)36(48-6)34(22-26)47-5)37(42)39-18-9-8-13-29(39)38(43)50-30(25-11-10-12-27(20-25)49-23-35(40)41)16-14-24-15-17-31(44-2)32(19-24)45-3/h10-12,15,17,19-22,28-30H,7-9,13-14,16,18,23H2,1-6H3,(H,40,41)/t28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132541

((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-butyryl]-pi...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C38H47NO11/c1-7-28(26-21-33(46-4)36(48-6)34(22-26)47-5)37(42)39-18-9-8-13-29(39)38(43)50-30(25-11-10-12-27(20-25)49-23-35(40)41)16-14-24-15-17-31(44-2)32(19-24)45-3/h10-12,15,17,19-22,28-30H,7-9,13-14,16,18,23H2,1-6H3,(H,40,41)/t28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132550

((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-propionyl]-...)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](C)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C37H45NO11/c1-23(26-20-32(45-4)35(47-6)33(21-26)46-5)36(41)38-17-8-7-12-28(38)37(42)49-29(25-10-9-11-27(19-25)48-22-34(39)40)15-13-24-14-16-30(43-2)31(18-24)44-3/h9-11,14,16,18-21,23,28-29H,7-8,12-13,15,17,22H2,1-6H3,(H,39,40)/t23-,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086078

(1-(2-Cyclohexyl-2-phenyl-acetyl)-piperidine-2-carb...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@H](C2CCCCC2)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H47NO8/c1-45-34-22-20-27(24-35(34)46-2)19-21-33(30-16-11-17-31(25-30)47-26-36(41)42)48-39(44)32-18-9-10-23-40(32)38(43)37(28-12-5-3-6-13-28)29-14-7-4-8-15-29/h3,5-6,11-13,16-17,20,22,24-25,29,32-33,37H,4,7-10,14-15,18-19,21,23,26H2,1-2H3,(H,41,42)/t32-,33?,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132558

((E)-(12S,13R,14S,17R,21S,23S,24R,25R,27R)-17-Ethyl...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC([C@H](C)C[C@H]2OC)C(=O)C(=O)N2CCCCC2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C43H69NO11/c1-10-30-18-24(2)17-25(3)19-36(52-8)41-37(53-9)21-27(5)40(54-41)38(48)42(49)44-16-12-11-13-31(44)43(50)55-39(28(6)33(46)23-34(30)47)26(4)20-29-14-15-32(45)35(22-29)51-7/h18,20,25,27-33,35-37,39-41,45-46H,10-17,19,21-23H2,1-9H3/b24-18+,26-20+/t25-,27+,28+,29-,30+,31?,32+,33-,35+,36-,37+,39+,40?,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132542
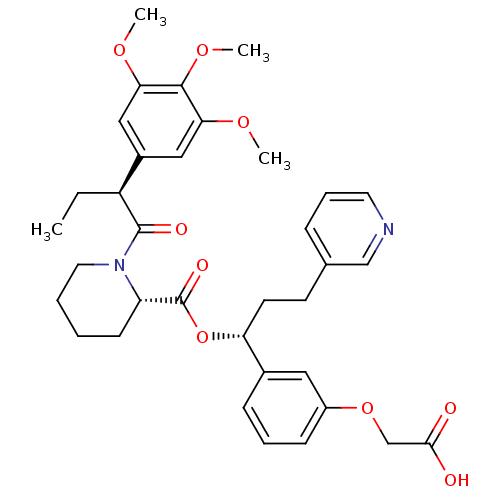
((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-butyryl]-pi...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1cccnc1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C35H42N2O9/c1-5-27(25-19-30(42-2)33(44-4)31(20-25)43-3)34(40)37-17-7-6-13-28(37)35(41)46-29(15-14-23-10-9-16-36-21-23)24-11-8-12-26(18-24)45-22-32(38)39/h8-12,16,18-21,27-29H,5-7,13-15,17,22H2,1-4H3,(H,38,39)/t27-,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086082
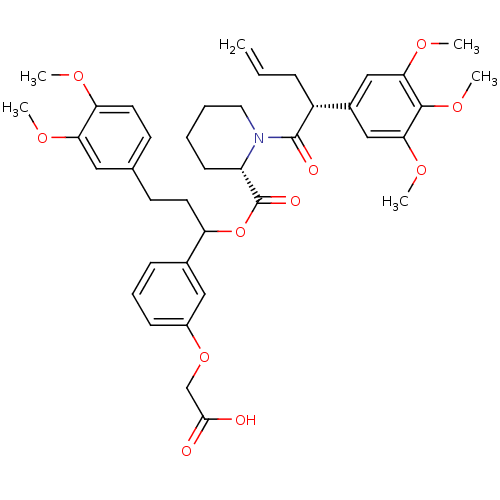
(1-[2-(3,4,5-Trimethoxy-phenyl)-pent-4-enoyl]-piper...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC=C)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H47NO11/c1-7-11-29(27-22-34(47-4)37(49-6)35(23-27)48-5)38(43)40-19-9-8-14-30(40)39(44)51-31(26-12-10-13-28(21-26)50-24-36(41)42)17-15-25-16-18-32(45-2)33(20-25)46-3/h7,10,12-13,16,18,20-23,29-31H,1,8-9,11,14-15,17,19,24H2,2-6H3,(H,41,42)/t29-,30+,31?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086087
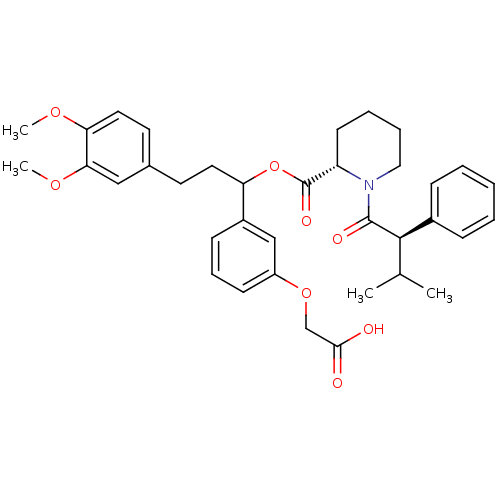
(1-(3-Methyl-2-phenyl-butyryl)-piperidine-2-carboxy...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@H](C(C)C)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C36H43NO8/c1-24(2)34(26-11-6-5-7-12-26)35(40)37-20-9-8-15-29(37)36(41)45-30(27-13-10-14-28(22-27)44-23-33(38)39)18-16-25-17-19-31(42-3)32(21-25)43-4/h5-7,10-14,17,19,21-22,24,29-30,34H,8-9,15-16,18,20,23H2,1-4H3,(H,38,39)/t29-,30?,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132560
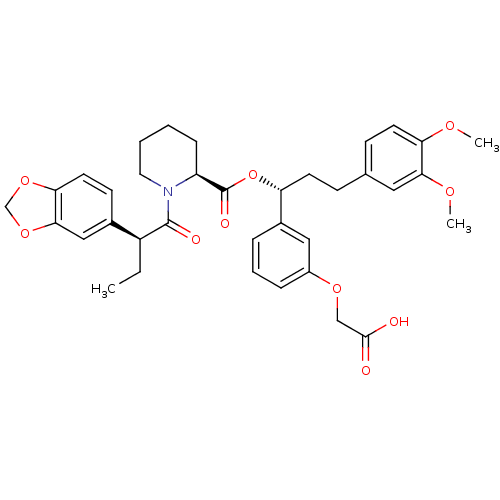
((S)-1-((S)-2-Benzo[1,3]dioxol-5-yl-butyryl)-piperi...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1ccc2OCOc2c1 Show InChI InChI=1S/C36H41NO10/c1-4-27(24-13-16-31-33(20-24)46-22-45-31)35(40)37-17-6-5-10-28(37)36(41)47-29(25-8-7-9-26(19-25)44-21-34(38)39)14-11-23-12-15-30(42-2)32(18-23)43-3/h7-9,12-13,15-16,18-20,27-29H,4-6,10-11,14,17,21-22H2,1-3H3,(H,38,39)/t27-,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086085
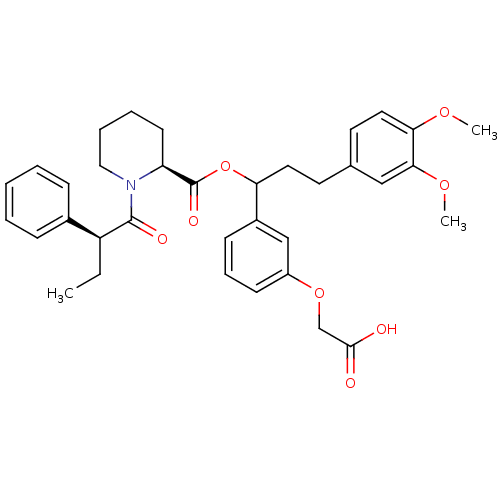
(1-(2-Phenyl-butyryl)-piperidine-2-carboxylic acid ...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)OC(CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1ccccc1 Show InChI InChI=1S/C35H41NO8/c1-4-28(25-11-6-5-7-12-25)34(39)36-20-9-8-15-29(36)35(40)44-30(26-13-10-14-27(22-26)43-23-33(37)38)18-16-24-17-19-31(41-2)32(21-24)42-3/h5-7,10-14,17,19,21-22,28-30H,4,8-9,15-16,18,20,23H2,1-3H3,(H,37,38)/t28?,29-,30?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132544
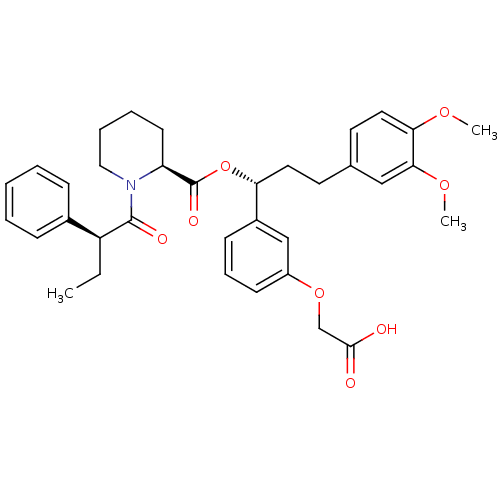
((S)-1-((S)-2-Phenyl-butyryl)-piperidine-2-carboxyl...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1ccccc1 Show InChI InChI=1S/C35H41NO8/c1-4-28(25-11-6-5-7-12-25)34(39)36-20-9-8-15-29(36)35(40)44-30(26-13-10-14-27(22-26)43-23-33(37)38)18-16-24-17-19-31(41-2)32(21-24)42-3/h5-7,10-14,17,19,21-22,28-30H,4,8-9,15-16,18,20,23H2,1-3H3,(H,37,38)/t28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132544

((S)-1-((S)-2-Phenyl-butyryl)-piperidine-2-carboxyl...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1ccccc1 Show InChI InChI=1S/C35H41NO8/c1-4-28(25-11-6-5-7-12-25)34(39)36-20-9-8-15-29(36)35(40)44-30(26-13-10-14-27(22-26)43-23-33(37)38)18-16-24-17-19-31(41-2)32(21-24)42-3/h5-7,10-14,17,19,21-22,28-30H,4,8-9,15-16,18,20,23H2,1-3H3,(H,37,38)/t28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086075
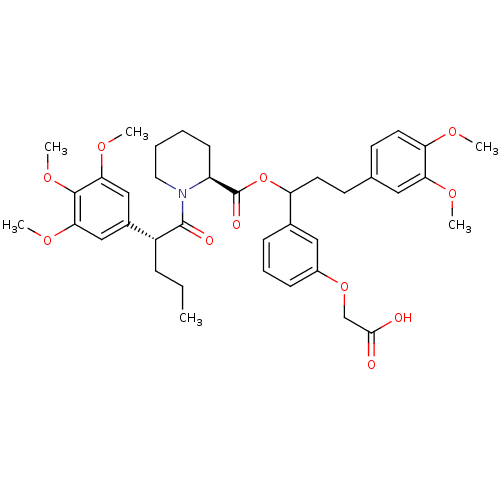
(1-[2-(3,4,5-Trimethoxy-phenyl)-pentanoyl]-piperidi...)Show SMILES CCC[C@@H](C(=O)N1CCCC[C@H]1C(=O)OC(CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C39H49NO11/c1-7-11-29(27-22-34(47-4)37(49-6)35(23-27)48-5)38(43)40-19-9-8-14-30(40)39(44)51-31(26-12-10-13-28(21-26)50-24-36(41)42)17-15-25-16-18-32(45-2)33(20-25)46-3/h10,12-13,16,18,20-23,29-31H,7-9,11,14-15,17,19,24H2,1-6H3,(H,41,42)/t29-,30+,31?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132556
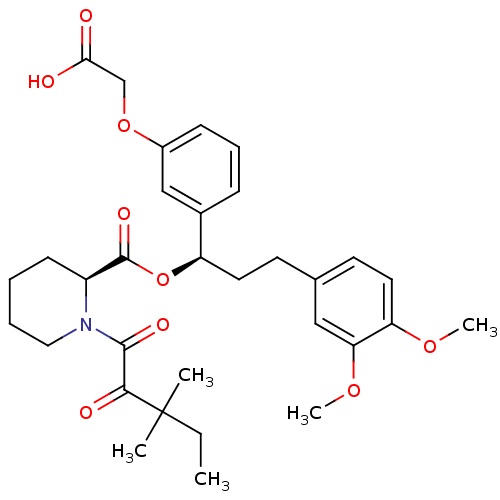
((S)-1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1 Show InChI InChI=1S/C32H41NO9/c1-6-32(2,3)29(36)30(37)33-17-8-7-12-24(33)31(38)42-25(22-10-9-11-23(19-22)41-20-28(34)35)15-13-21-14-16-26(39-4)27(18-21)40-5/h9-11,14,16,18-19,24-25H,6-8,12-13,15,17,20H2,1-5H3,(H,34,35)/t24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132551
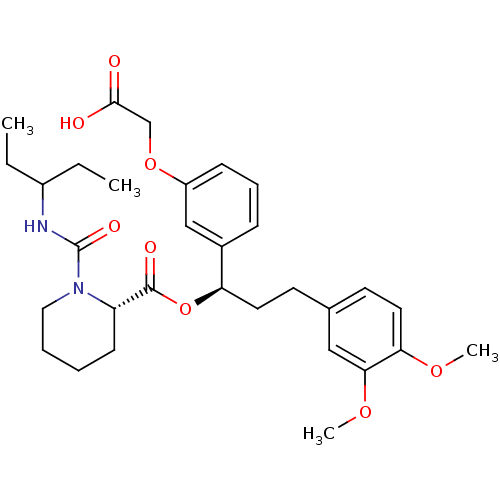
(1-(1-Ethyl-propylcarbamoyl)-piperidine-2-carboxyli...)Show SMILES CCC(CC)NC(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1 Show InChI InChI=1S/C31H42N2O8/c1-5-23(6-2)32-31(37)33-17-8-7-12-25(33)30(36)41-26(22-10-9-11-24(19-22)40-20-29(34)35)15-13-21-14-16-27(38-3)28(18-21)39-4/h9-11,14,16,18-19,23,25-26H,5-8,12-13,15,17,20H2,1-4H3,(H,32,37)(H,34,35)/t25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086086
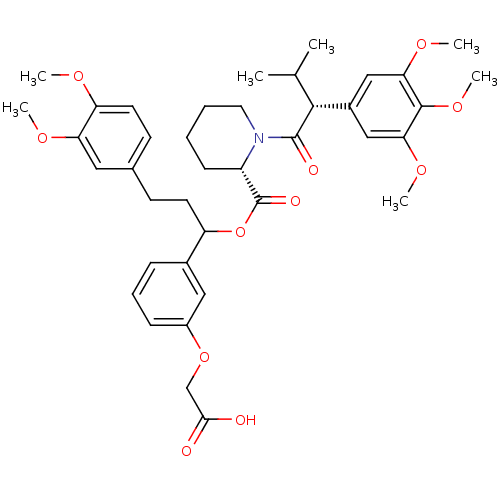
(1-[3-Methyl-2-(3,4,5-trimethoxy-phenyl)-butyryl]-p...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@H](C(C)C)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H49NO11/c1-24(2)36(27-21-33(47-5)37(49-7)34(22-27)48-6)38(43)40-18-9-8-13-29(40)39(44)51-30(26-11-10-12-28(20-26)50-23-35(41)42)16-14-25-15-17-31(45-3)32(19-25)46-4/h10-12,15,17,19-22,24,29-30,36H,8-9,13-14,16,18,23H2,1-7H3,(H,41,42)/t29-,30?,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086079
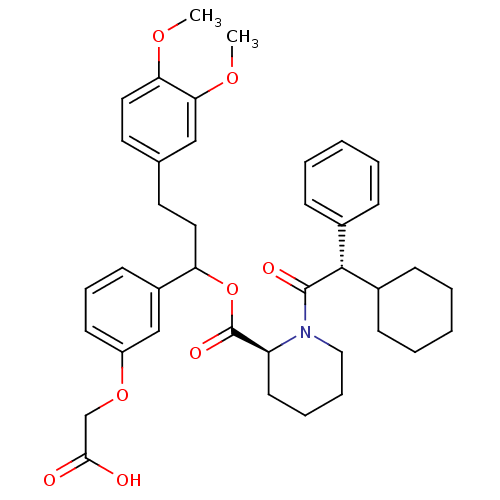
(1-(2-Cyclohexyl-2-phenyl-acetyl)-piperidine-2-carb...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](C2CCCCC2)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H47NO8/c1-45-34-22-20-27(24-35(34)46-2)19-21-33(30-16-11-17-31(25-30)47-26-36(41)42)48-39(44)32-18-9-10-23-40(32)38(43)37(28-12-5-3-6-13-28)29-14-7-4-8-15-29/h3,5-6,11-13,16-17,20,22,24-25,29,32-33,37H,4,7-10,14-15,18-19,21,23,26H2,1-2H3,(H,41,42)/t32-,33?,37+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086080
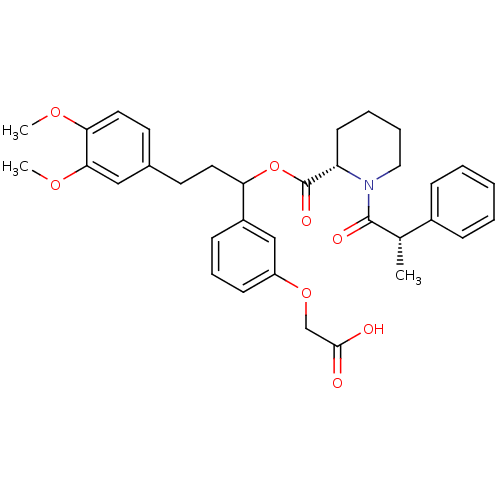
(1-(2-Phenyl-propionyl)-piperidine-2-carboxylic aci...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](C)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C34H39NO8/c1-23(25-10-5-4-6-11-25)33(38)35-19-8-7-14-28(35)34(39)43-29(26-12-9-13-27(21-26)42-22-32(36)37)17-15-24-16-18-30(40-2)31(20-24)41-3/h4-6,9-13,16,18,20-21,23,28-29H,7-8,14-15,17,19,22H2,1-3H3,(H,36,37)/t23-,28-,29?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086089
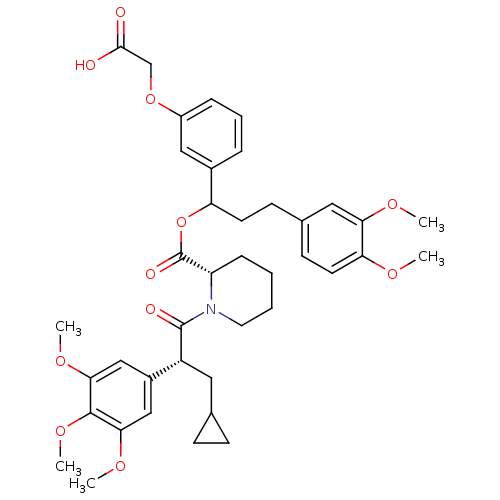
(1-[3-Cyclopropyl-2-(3,4,5-trimethoxy-phenyl)-propi...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC2CC2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C40H49NO11/c1-46-33-17-15-26(20-34(33)47-2)14-16-32(27-9-8-10-29(21-27)51-24-37(42)43)52-40(45)31-11-6-7-18-41(31)39(44)30(19-25-12-13-25)28-22-35(48-3)38(50-5)36(23-28)49-4/h8-10,15,17,20-23,25,30-32H,6-7,11-14,16,18-19,24H2,1-5H3,(H,42,43)/t30-,31+,32?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
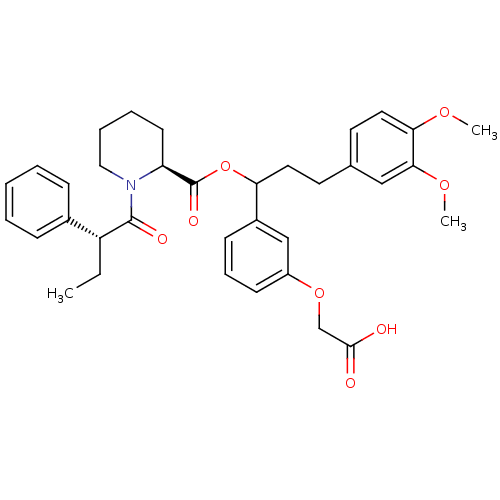
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086084
(1-(2-Phenyl-butyryl)-piperidine-2-carboxylic acid ...)Show SMILES CC[C@@H](C(=O)N1CCCC[C@H]1C(=O)OC(CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1ccccc1 Show InChI InChI=1S/C35H41NO8/c1-4-28(25-11-6-5-7-12-25)34(39)36-20-9-8-15-29(36)35(40)44-30(26-13-10-14-27(22-26)43-23-33(37)38)18-16-24-17-19-31(41-2)32(21-24)42-3/h5-7,10-14,17,19,21-22,28-30H,4,8-9,15-16,18,20,23H2,1-3H3,(H,37,38)/t28-,29+,30?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
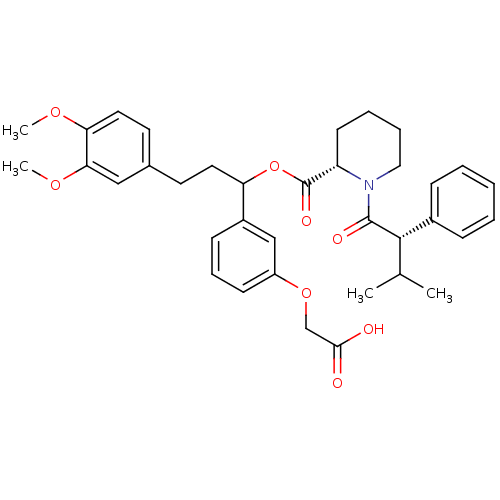
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086081
(1-(3-Methyl-2-phenyl-butyryl)-piperidine-2-carboxy...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](C(C)C)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C36H43NO8/c1-24(2)34(26-11-6-5-7-12-26)35(40)37-20-9-8-15-29(37)36(41)45-30(27-13-10-14-28(22-27)44-23-33(38)39)18-16-25-17-19-31(42-3)32(21-25)43-4/h5-7,10-14,17,19,21-22,24,29-30,34H,8-9,15-16,18,20,23H2,1-4H3,(H,38,39)/t29-,30?,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
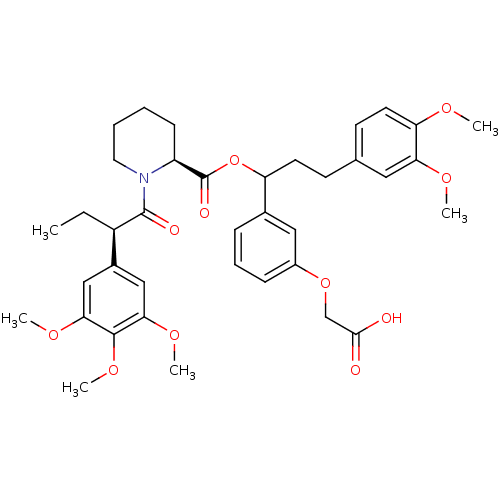
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086093
(1-[2-(3,4,5-Trimethoxy-phenyl)-butyryl]-piperidine...)Show SMILES CC[C@@H](C(=O)N1CCCC[C@H]1C(=O)OC(CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C38H47NO11/c1-7-28(26-21-33(46-4)36(48-6)34(22-26)47-5)37(42)39-18-9-8-13-29(39)38(43)50-30(25-11-10-12-27(20-25)49-23-35(40)41)16-14-24-15-17-31(44-2)32(19-24)45-3/h10-12,15,17,19-22,28-30H,7-9,13-14,16,18,23H2,1-6H3,(H,40,41)/t28-,29+,30?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086076
(1-Diphenylacetyl-piperidine-2-carboxylic acid 1-(3...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)C(c2ccccc2)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H41NO8/c1-45-34-22-20-27(24-35(34)46-2)19-21-33(30-16-11-17-31(25-30)47-26-36(41)42)48-39(44)32-18-9-10-23-40(32)38(43)37(28-12-5-3-6-13-28)29-14-7-4-8-15-29/h3-8,11-17,20,22,24-25,32-33,37H,9-10,18-19,21,23,26H2,1-2H3,(H,41,42)/t32-,33?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data